Advertisements
Advertisements
Question
M is a bivalent metal. Write down the steps to find the chemical formulae of its compounds formed with the radicals, sulphate and phosphate.
Solution
The symbols and valencies of M and sulphate:
| symbols | M | SO4 |
| valencies | 2 | 2 |
Cross-multiply the valencies
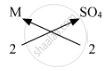
The chemical formula is MSO4.
The symbols and valencies of M and phosphate:
| symbols | M | PO4 |
| valencies | 2 | 3 |
Cross-multiply the valencies
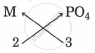
The chemical formula is M3(PO4)2.
APPEARS IN
RELATED QUESTIONS
Write the steps in deducing the chemical formulae of the following compound.
Sodium sulphate
Write the steps in deducing the chemical formulae of the following compound.
Potassium nitrate
Write the steps in deducing the chemical formulae of the following compound.
Ferric phosphate
Write the steps in deducing the chemical formulae of the following compound.
Calcium oxide
Write the steps in deducing the chemical formulae of the following compound.
Aluminium hydroxide
