Advertisements
Advertisements
Question
Match the following reactants in Column I with the corresponding reaction products in Column II.
| Column I | Column II |
| (i) \[\ce{Benzene + Cl2 ->[AlCl3]}\] | (a) Benzoic acid |
| (ii) \[\ce{Benzene + CH3Cl ->[AlCl3]}\] | (b) Methyl phenyl ketone |
| (iii) \[\ce{Benzene + CH3COCl ->[AlCl3]}\] | (c) Toluene |
| (iv) \[\ce{Toluene ->[KMnO4/NaOH]}\] | (d) Chlorobenzene |
| (e) Benzene hexachloride |
Solution
| Column I | Column II |
| (i) \[\ce{Benzene + Cl2 ->[AlCl3]}\] | (d) Chlorobenzene |
| (ii) \[\ce{Benzene + CH3Cl ->[AlCl3]}\] | (c) Toluene |
| (iii) \[\ce{Benzene + CH3COCl ->[AlCl3]}\] | (b) Methyl phenyl ketone |
| (iv) \[\ce{Toluene ->[KMnO4/NaOH]}\] | (a) Benzoic acid |
APPEARS IN
RELATED QUESTIONS
How will you convert benzene into p-nitrobromobenzene?
How would you convert the following compound into benzene?
Ethyne
How would you convert the following compound into benzene?
Ethene
How would you convert the following compound into benzene?
Hexane
What will be the product obtained as a result of the following reaction and why?
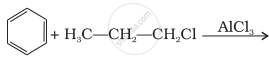
Suggest a route for the preparation of nitrobenzene starting from acetylene?
On heating the mixture of sodium benzoate and soda-lime, the product obtained is ______.
Preparation of benzene from phenol is:
Benzene reacts with benzoyl chloride to form ______.
Phenol associates in benzene to a certain extent to form a dimer. A solution containing 20 × 10-3 kg of phenol in 1.0 kg of benzene has its freezing point depressed by 0.69 K The fraction of phenol that has dimerised is ______. (Kf for benzene = 5.12 K kg mol-1)
