Advertisements
Advertisements
Question
Match the hydrocarbons in Column I with the boiling points given in Column II.
| Column I | Column II |
| (i) n-Pentane | (a) 282.5 K |
| (ii) iso-Pentane | (b) 309 K |
| (iii) neo-Pentane | (c) 301 K |
Solution
| Column I | Column II |
| (i) n-Pentane | (b) 309 K |
| (ii) iso-Pentane | (c) 301 K |
| (iii) neo-Pentane | (a) 282.5 K |
APPEARS IN
RELATED QUESTIONS
The increasing order of reduction of alkyl halides with zinc and dilute HCl is ______.
Write the structures and names of products obtained in the reactions of sodium with a mixture of 1-iodo-2-methylpropane and 2-iodopropane.
896 mL vapour of a hydrocarbon ‘A’ having carbon 87.80% and hydrogen 12.19% weighs 3.28 g at STP. Hydrogenation of ‘A’ gives 2-methylpentane. Also ‘A’ on hydration in the presence of H2SO4 and HgSO4 gives a ketone ‘B’ having molecular formula C6H12O. The ketone ‘B’ gives a positive iodoform test. Find the structure of ‘A’ and give the reactions involved.
An unsaturated hydrocarbon ‘A’ adds two molecules of H2 and on reductive ozonolysis gives butane-1,4-dial, ethanal and propanone. Give the structure of ‘A’, write its IUPAC name and explain the reactions involved.
An alkyl halide by formation of its Grignard reagent and heating with water gives propane. What is the original alkyl halide?
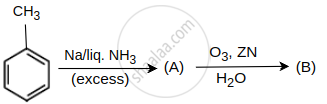
Identify products A and B.

In the given reaction,
\[\ce{2-Bromo-3, 3-dimethyl butane ->[C2H5OH][\underset{(Major product)}{'A'}]}\] Product A is ______.
Which of the following is Lindlar's catalyst?
The number of nitrogen atoms in a semicarbazone molecule of acetone is ______.
In the given reaction final product(s) will be: