Advertisements
Advertisements
Question
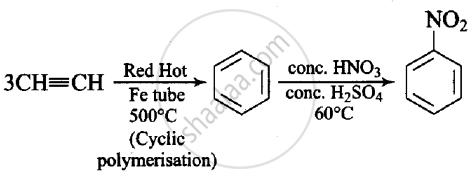
Suggest a route for the preparation of nitrobenzene starting from acetylene?
Solution
Acetylene when passed through red hot iron tube at 500°C undergoes cyclic polymerisation to give benzene which upon nitration gives nitrobenzene.
APPEARS IN
RELATED QUESTIONS
How will you convert benzene into p-nitrobromobenzene?
How would you convert the following compound into benzene?
Ethyne
How would you convert the following compound into benzene?
Ethene
How would you convert the following compound into benzene?
Hexane
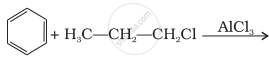
What will be the product obtained as a result of the following reaction and why?
Match the following reactants in Column I with the corresponding reaction products in Column II.
| Column I | Column II |
| (i) \[\ce{Benzene + Cl2 ->[AlCl3]}\] | (a) Benzoic acid |
| (ii) \[\ce{Benzene + CH3Cl ->[AlCl3]}\] | (b) Methyl phenyl ketone |
| (iii) \[\ce{Benzene + CH3COCl ->[AlCl3]}\] | (c) Toluene |
| (iv) \[\ce{Toluene ->[KMnO4/NaOH]}\] | (d) Chlorobenzene |
| (e) Benzene hexachloride |
On heating the mixture of sodium benzoate and soda-lime, the product obtained is ______.
Preparation of benzene from phenol is:
Benzene reacts with benzoyl chloride to form ______.
Phenol associates in benzene to a certain extent to form a dimer. A solution containing 20 × 10-3 kg of phenol in 1.0 kg of benzene has its freezing point depressed by 0.69 K The fraction of phenol that has dimerised is ______. (Kf for benzene = 5.12 K kg mol-1)
