Advertisements
Advertisements
Question
Prove the law of conservation of energy for a particle performing simple harmonic motion.Hence graphically show the variation of kinetic energy and potential energy w. r. t. instantaneous displacement.
Solution
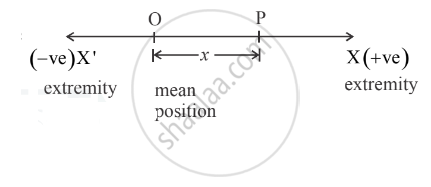
i. Suppose a particle of mass m performing linear S.H.M. is at point P which is at a distance x
from the mean position O as shown in figure.
ii. Kinetic energy of particle at point P is given by,
`K.E=1/2momega^2(A^2-x^2)`
iii. Potential energy at point P is given by,
`P.E=1/2momega^2x^2`
iv. Total energy at point P is given by,
T.E. = K.E. + P.E
`=1/2 momega^2(A^2-x^2)+1/2momega^2x^2`
`=1/2momega^2(A^2-x^2+x^2)`
`T.E=1/2momega^2A^2`............(1)
v. If particle is at mean position:
x = 0
`therefore K.E=1/2 momega^2A^2`
`P.E=1/2momega^2xx0=0`
`therefore T.E=K.E+P.E=1/2momega^2A^2`...............(2)
vi. If particle is at extreme position:
x = A
`K.E=1/2momega^2(A^2-A^2)=0`
`P.E=1/2 momega^2A^2`
`therefore T.E=P.E+K.E=1/2 momega^2A^2`...............(3)
vii. From equations (1), (2) and (3), it is observed that total energy of a particle performing linear S.H.M. at any point in its path is constant. Hence, total energy of linear S.H.M. remains conserved.
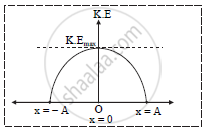
viii. a. Graph of variation of kinetic energy w. r. t. instantaneous displacement.
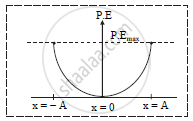
b. Graph of variation of potential energy w.r.t. instantaneous displacement
APPEARS IN
RELATED QUESTIONS
Calculate the average molecular kinetic energy :
(a) per kilomole, (b) per kilogram, of oxygen at 27°C.
(R = 8320 J/k mole K, Avogadro's number = 6*03 x 1026 molecules/K mole)
Obtain an expression for potential energy of a particle performing simple harmonic motion. Hence evaluate the potential energy
- at mean position and
- at extreme position.
State an expression for K. E. (kinetic energy) and P. E. (potential energy) at displacement ‘x’ for a particle performing linear S.H. M. Represent them graphically. Find the displacement at which K. E. is equal to P. E.
The kinetic energy of nitrogen per unit mass at 300 K is 2.5 × 106 J/kg. Find the kinetic energy of 4 kg oxygen at 600 K. (Molecular weight of nitrogen = 28, Molecular weight of oxygen = 32)
Obtan an expression for potential energy of a particle performing S.H.M. What is the value of potential energy at (i) Mean position, and (ii) Extreme position
