Advertisements
Advertisements
Question
Give the resonating structures of NO2 and N2O5.
Solution
NO2 :-
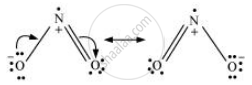
N2O5:-

APPEARS IN
RELATED QUESTIONS
Pb(NO3)2 on heating gives a brown gas which undergoes dimerisation on cooling? Identify the gas
Why does NO2 dimerise?
What is the covalence of nitrogen in N2O5?
Why does the reactivity of nitrogen differ from phosphorus?
How is nitrogen prepared in the laboratory? Write the chemical equations of the reactions involved.
Which of the following is a basic oxide?
(A) SiO2
(B) P4O10
(C) MgO
(D) Al2O3
Explain why Mn2+ ion is more stable than Mn3+? (Given : Mn→ Z=25)
Account for the following :
Bond angle in NH+4NH4+ is higher than NH3.
Tendency to show –3 oxidation state decrease from Nitrogn (N) to Bismuth (Bi).
The statement true for \[\ce{N^-_3}\] is ____________.
Which of the following statements is not correct for nitrogen?
Nitrogen is relatively inactive element because ____________.
Among the 15th group elements, as we move from nitrogen to bismuth, the pentavalency becomes less pronounced and trivalency becomes more pronounced due to:
The bonds present in N2O5 are ____________.
Which of the following oxides of nitrogen reacts with FeSO4 to form a dark brown compound?
On heating lead nitrate forms oxides of nitrogen and lead. The oxides formed are ______.
Assertion: \[\ce{HNO3}\] makes iron passive.
Reason: \[\ce{HNO3}\] forms a protective layer of ferric nitrate on the surface of iron.
Heating white phosphorus with conc. NaOH solution gives mainly ______.
