Advertisements
Advertisements
Question
Saloni took a piece of burning charcoal and collected the gas evolved in a test tube.
(a) How will she find the nature of the gas?
(b) Write down word equations of all the reactions taking place in this process.
Solution
(a) Add a few drops of water in the test tube containing gas. Now, cover the test tube and shake it well. After shaking, test the solution with blue litmus and red litmus. It will turn blue litmus red. Thus, the gas is acidic in nature.
(b) Charcoal reacts with oxygen to form carbon dioxide gas.

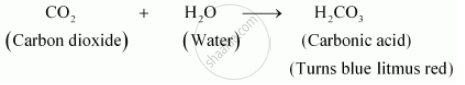
Carbon dioxide reacts with water to form carbonic acid, which turns blue litmus paper red.
APPEARS IN
RELATED QUESTIONS
Mark ‘T’ if the statement is true and ‘F’ if it is false.
Sodium is a very reactive metal. ( )
What is an alloy? How is an alloy made?
Statements given below are incorrect. Write the correct statements :
Bronze is a mixture of 10% copper, 80% zinc and 10% tin.
State the position of the following in the periodic table:
Alkali metals
Write name.
The nonmetal having electrical conductivity.
When a copper coin is dipped in silver nitrate solution, a glitter appears on the coin after some time. Why does this happen? Write the chemical equation.
What is it made from? Why?
Ornaments
The minerals from which the metal can be separated economically are called _______.
Why are bells made of metals?
Sodium is a hard metal.
