Advertisements
Advertisements
Question
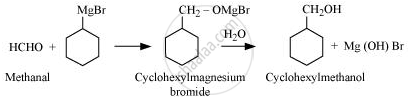
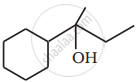
Show how is the following alcohol prepared by the reaction of a suitable Grignard reagent on methanal?
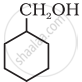
Solution
APPEARS IN
RELATED QUESTIONS
How will you convert: Propene to Propan-2-ol?
Write the main product(s) in each of the following reactions:
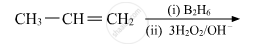
How do you convert the following : Propan-2-ol to 2-methylpropan-2-ol
Show how is the following alcohol prepared by the reaction of a suitable Grignard reagent on methanal?
\[\begin{array}{cc}
\ce{CH3 - CH - CH2OH}\\
|\phantom{......}\\
\ce{CH3}\phantom{...}
\end{array}\]
Write the mechanism of hydration of ethene to yield ethanol.
Show how you would synthesise the following alcohol from an appropriate alkene?
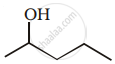
Show how you would synthesise the following alcohol from an appropriate alkene?
Write the structure of main compounds A and B in the following reaction:
\[\ce{CH3CH2CN->[CH3MgBRH/3O+]A->[LiAIH4]B}\]
Primary alcohols are prepared by the reduction of carboxylic acids. Though lithium aluminium hydride is a strong reducing agent, it is not used in the reaction. This is so because:
Identify ‘C’ in the following:
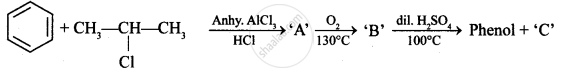
Why is the reactivity of all the three classes of alcohols with conc. \[\ce{HCl}\] and \[\ce{ZnCl2}\] (Lucas reagent) different?
The reagent used for dehydration of an alcohol is
The best reagent to convert pent 3 – en 2 – 01 into pent 3 – in – 2 – one is
When alcohol react with concentrated H2SO4 intermediate compound formed is
The best reagent to convert pent - 3 - en - 2 - ol into pent - 3 - en - 2 one is ______.
To synthesise 1.0 mole of 2-methylpropan-2-ol from Ethylethanoate ______ equivalents of CH3MgBr reagent will be required. (Integer value)
An aldehyde isomeric with allyl alcohol gives phenyl hydrazone. Pick out a ketone that too gives a phenyl hydrazone containing the same percentage of nitrogen.
The major product of the following reaction is:
\[\begin{array}{cc}
\ce{Cl}\phantom{.........................}\\
|\phantom{..........................}\\
\ce{CH3 - CH - CH3 ->[(i) Alc. KOH][(ii) HBr/peroxide (iii) aq. KOH]}
\end{array}\]
Given below are two statements:
Statement I: On heating with KHSO4, glycerol is dehydrated and acrolein is formed.
Statement II: Acrolein has a fruity odour and can be used to test glycerol's presence.
Choose the correct option.
Write the mechanism of acid dehydration of ethanol to yield ethene.
