Advertisements
Advertisements
Question
Solve the following problem:
Perform the following calculation. Round off your answer to three digits.
`((9.28xx10^9)(9.9xx10^-7))/((511)(2.98xx10^-6))`
Solution
`((9.28xx10^9)(9.9xx10^-7))/((511)(2.98xx10^-6))`
= `0.06033 × 10^(9–7–(–6))`
= 0.06033 × 108
= 6.03 × 106
APPEARS IN
RELATED QUESTIONS
If the density of methanol is 0.793 kg L–1, what is its volume needed for making 2.5 L of its 0.25 M solution?
Dinitrogen and dihydrogen react with each other to produce ammonia according to the following chemical equation:
\[\ce{N2 (g) + 3H2 (g) → 2NH3 (g)}\]
(i) Calculate the mass of ammonia produced if 2.00 × 103 g dinitrogen reacts with 1.00 × 103 g of dihydrogen.
(ii) Will any of the two reactants remain unreacted?
(iii) If yes, which one and what would be its mass?
Calculate the molarity of a solution of ethanol in water in which the mole fraction of ethanol is 0.040 (assume the density of water to be one).
To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Explain the term mole fraction
Explain the term molality
Solve the following problem:
Find out the molar mass of the following compounds:
Sodium carbonate, decahydrate (Na2CO3.10H2O)
(At. mass: Cu = 63.5; S = 32; O = 16; H = 1; Na = 23; C = 12; Fe = 56; N = 14)
Solve the following problem:
Find out the molar mass of the following compounds:
Mohr’s salt [FeSO4(NH4)2SO4.6H2O]
(At. mass: Cu = 63.5; S = 32; O = 16; H = 1; Na = 23; C = 12; Fe = 56; N = 14)
Solve the following problem:
Write the following number in ordinary decimal form:
14.3 × 10−2
Solve the following problem:
Write the following number in ordinary decimal form:
5.00858585
Solve the following problem:
Perform the following calculation. Round off your answer to two digits.
`(1.4xx10^9)/((2.77xx10^3)(3.76xx10^5))`
Solve the following problem:
Perform the following calculation. Round off your answer to two digits.
`((4xx10^-3)(9.9xx10^-7))/((789)(1.002xx10^-10)(0.3xx10^2))`
Perform each of the following calculations. Round off your answers to three digits.
(3.26104) (1.54106)
Solve the following problem:
A 1.000 mL sample of acetone, a common solvent used as a paint remover, was placed in a small bottle whose mass was known to be 38.0015 g.
The following values were obtained when the acetone - filled bottle was weighed: 38.7798 g, 38.7795 g and 38.7801 g. How would you characterise the precision and accuracy of these measurements if the actual mass of the acetone was 0.7791 g?
Solve the following problem:
Your laboratory partner was given the task of measuring the length of a box (approx 5 in) as accurately as possible, using a metre stick graduated in milimeters. He supplied you with the following measurements:
12.65 cm, 12.6 cm, 12.65 cm, 12.655 cm, 126.55 mm, 12 cm.
Give your reason for rejecting each of the others.
What are the favourable qualities given to gold when it is alloyed with copper or silver for the purpose of making ornaments?
When light is passed through water containing a few drops of milk, it shows a bluish tinge. This is due to the ______ of light by milk and the phenomenon is called ______. This indicates that milk is a ______ solution.
Non-metals are usually poor conductors of heat and electricity. They are non-lustrous, non-sonorous, non-malleable and are coloured.
Name a lustrous non-metal.
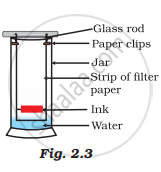
A child wanted to separate the mixture of dyes constituting a sample of ink. He marked a line by the ink on the filter paper and placed the filter paper in a glass containing water as shown in Fig.2.3. The filter paper was removed when the water moved near the top of the filter paper.
(i) What would you expect to see, if the ink contains three different coloured components?
(ii) Name the technique used by the child.
(iii) Suggest one more application of this technique.
Sulphuric acid reacts with sodium hydroxide as follows:
\[\ce{H2SO4 + 2NaOH -> Na2SO4 + 2H2O}\]
When 1 L of 0.1 M sulphuric acid solution is allowed to react with 1 L of 0.1 M sodium hydroxide solution, the amount of sodium sulphate formed and its molarity in the solution obtained is:
(i) 0.1 mol L–1
(ii) 7.10 g
(iii) 0.025 mol L–1
(iv) 3.55 g
If 4 g of \[\ce{NaOH}\] dissolves in 36 g of \[\ce{H2O}\], calculate the mole fraction of each component in the solution. Also, determine the molarity of solution (specific gravity of solution is 1g mL–1).
Match the following physical quantities with units
| Physical quantity | Unit |
| (i) Molarity | (a) g mL–1 |
| (ii) Mole fraction | (b) mol |
| (iii) Mole | (c) Pascal |
| (iv) Molality | (d) Unitless |
| (v) Pressure | (e) mol L–1 |
| (vi) Luminous intensity | (f) Candela |
| (vii) Density | (g) mol kg–1 |
| (viii) Mass | (h) Nm–1 |
| (i) kg |
An aqueous KCl solution of density 1.20 g mL-1 has a molality of 3.30 mol kg-1. The molarity of the solution in mol L-1 is ______. (Nearest integer)
What quantity (in mL) of a 45% acid solution of a monoprotic strong acid must be mixed with a 20% solution of the same acid to produce 800 mL of a 29.875% acid solution?
The molarity of urea (molar mass 60 g mol−1) solution by dissolving 15 g of urea in 500 cm3 of water is ______.
Molarity is ______.
Find the molality of solution if boiling point increases by 1.75 K and molal elevation constant of solvent is 5K kg mol-1.
