Advertisements
Advertisements
Question
Some metals react with acids to produce salt and hydrogen gas. Illustrate it with an example. How will you test the presence of this gas?
Solution
Some metals react with acids to produce salt and hydrogen gas. For example, when magnesium reacts with hydrochloric acid, magnesium chloride (a salt) and hydrogen gas are produced. The chemical equation for this reaction is:
\[\ce{Mg + 2HCL -> MgCl2 + H2}\]
To test for the presence of hydrogen gas, bring a burning match or candle near the mouth of the reacting test tube. If hydrogen gas is present, it will ignite with a 'pop' sound, characteristic of hydrogen gas. This is because hydrogen gas is highly flammable and reacts with oxygen in the air to produce water vapour when ignited, making a popping sound.
APPEARS IN
RELATED QUESTIONS
| Column A | Column B | ||
| i | eosin | 1 | losing hydrogen |
| ii | oxidation | 2 | synthetic indicator |
| 3 | losing oxygen | ||
| 4 | natural indicator |
What is the chemical formula of baking soda?
Write the chemical formula of sodium carbonate decahydrate.
What is the chemical name of bleaching powder?
State one use of bleaching powder (other than bleaching).
Choose the correct alternative and rewrite the following sentence.
Phenolphthalein is ___________ type of indicator.
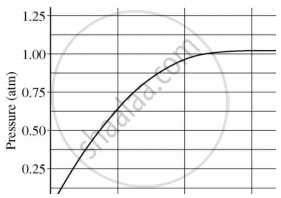
A student added 10 g of calcium carbonate in a rigid container, secured it tightly and started to heat it. After some time, an increase in pressure was observed, the pressure reading was then noted at intervals of 5 mins and plotted against time, in a graph as shown below. During which time interval did maximum decomposition take place?
A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange. Which of the following would change the colour of this pH paper to greenish-blue?
What happens when nitric acid is added to egg shell?
The taste of acid is ______.
