Advertisements
Advertisements
Question
State whether the given chemical reaction is a redox reaction or not. Justify your answer.
\[\ce{MnO_2 + 4HCl->MnCl_2 +2H_2O + Cl_2}\]
Solution
The given reaction is an example of an oxidation-reduction reaction or redox reaction.
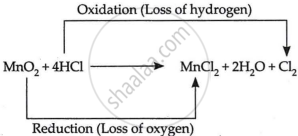
A substance is oxidised when it receives or loses hydrogen during a process. A material is decreased when it loses oxygen or absorbs hydrogen during a process. HCl loses hydrogen in the proposed process to generate Cl2. The provided reaction is thus a redox reaction.
RELATED QUESTIONS
When a magnesium ribbon burns in air with a dazzling flame and forms a white ash, is magnesium oxidised or reduced? Why?
Give one example of an oxidation-reduction reaction which is also a combination reaction.
In the reaction represented by the following equation:
CuO (s) + H2 (g) → Cu (s) + H2O (1)
(a) name the substance oxidised
(b) name the substance reduced
(c) name the oxidising agent
(d) name the reducing agent
Divide the following reactions into oxidation and reduction half-reaction:
Zn + Cu2+ → Cu + Zn 2+
Name:
an oxidizing agent that does not contain oxygen.
Identify the reducing agent in the following reactions
`4"NH"_3 + 5"O"_2 -> 4"NO" + 6"H"_2"O"`
Which among the following are physical or chemical change?
Evaporation of petrol
During the reaction of some metals with dilute hydrochloric acid, following observations are made.
Some bubbles of a gas are seen when lead (Pb) is reacted with the acid.
Explain this observation giving suitable reason.
Mention some oxidation reactions that occur in daily life.
What is rancidity?
