Advertisements
Advertisements
प्रश्न
State whether the given chemical reaction is a redox reaction or not. Justify your answer.
\[\ce{MnO_2 + 4HCl->MnCl_2 +2H_2O + Cl_2}\]
उत्तर
The given reaction is an example of an oxidation-reduction reaction or redox reaction.
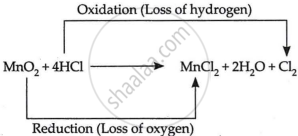
A substance is oxidised when it receives or loses hydrogen during a process. A material is decreased when it loses oxygen or absorbs hydrogen during a process. HCl loses hydrogen in the proposed process to generate Cl2. The provided reaction is thus a redox reaction.
संबंधित प्रश्न
Fill in the following blanks with suitable words:
The addition of oxygen to a substance is called ......... whereas removal of oxygen is called ........
Fill in the blank
In redox reaction oxidation and reduction occur ............................
How would you change a metal like Cu into its ions?
State whether the following conversion is oxidation or reduction:
PbO2 + SO2→ PbSO4
Divide the following reactions into oxidation and reduction half-reaction:
Zn + Cu2+ → Cu + Zn 2+
State what is oxidising agent. Give an example of oxidising agent in the gaseous, liquid, and solid form.
Which among the following are physical or chemical change?
Sublimation of solid ammonium chloride
Identify the following reactions as oxidation/reduction/redox reaction
Zn + CuSO4 → Cu + ZnSO4
Find the oxidation number of the element in the following compound.
Mg in MgO
It is recommended to use air tight containers for storing oil for a long time.
