Advertisements
Advertisements
Question
The beaker is divided into two chambers A and B. The big circle represents solute and the small circles solvent.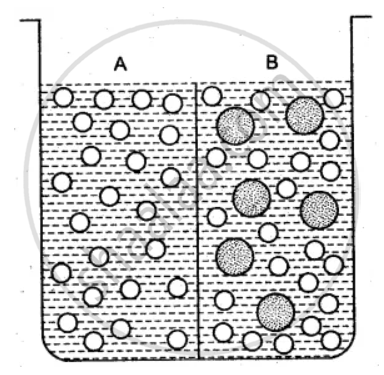
(i) What can you say about the size of the holes in the membrane, if it is to behave semi- permeably between these two?
(ii) Will the solvent molecules pass through the membrane from left to right, from right to left, in either direction or in both directions?
(iii) In which direction will there be a net movement of solvent molecules?
Solution
(i) The size of the holes in the membrane is large enough to allow only the solvent particles to pass through it. But solute particle cannot pass through it. Thus, the membrane acts as the semi-permeable.
(ii) Solvent molecules will pass through the membrane in both directions. Since solvent molecules are present on both the sides they will strike the semi-permeable membrane and pass through the same.
(iii) There is a net movement of solvent molecules from the place of its higher chemical potential to the place of its lower chemical potential, i.e., from right to left.
APPEARS IN
RELATED QUESTIONS
A plant cell placed in a certain solution got plasmolysed. What was the kind of solution?
Osmosis and diffusion are the same except that osmosis there is:
What is responsible for guttation?
Give the equivalent terms for the following:
Pressure of the cell contents on the cell wall
When placed in a more concentrated solution, the cell contents will ______.
Mention whether the following statement is true or false. Correct the false statement by altering the last word only.
Addition of salt to pickles prevents growth of bacteria because they turn turgid.
A leaf cell of a water plant was placed in a liquid other than pond water. After sometime, it assumed a shape as shown below:
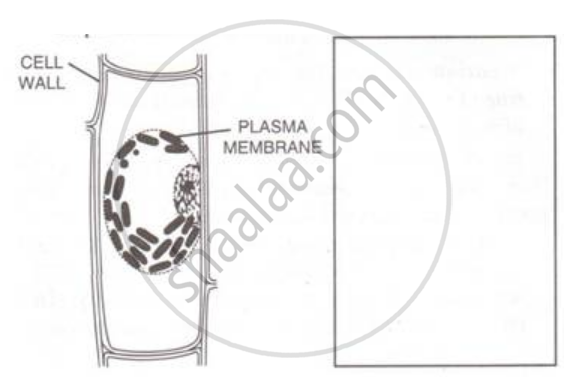
Redraw in the space provided, the diagram of the cell if it is soon placed in ordinary water for some time.
Differentiate between the following
Endosmosis and Exosmosis
Multiple Choice Question:
When a plant wilts, the sequence of events will be as follows:
Define the following term:
Osmotic Pressure
