Advertisements
Advertisements
Question
The edge lengths of the unit cells in terms of the radius of spheres constituting fcc, bcc and simple cubic unit cell are respectively ______.
Options
`2sqrt(2)r, (4r)/sqrt(3), 2r`
`(4r)/sqrt(3), 2sqrt(2)r, 2r`
`2r, 2sqrt(2)r, (4r)/sqrt(3)`
`2r, (4r)/sqrt(3), 2sqrt(2)r`
Solution
The edge lengths of the unit cells in terms of the radius of spheres constituting fcc, bcc and simple cubic unit cell are respectively `2sqrt(2)r, (4r)/sqrt(3), 2r`.
Explanation:
Note: Distance between two atoms is always measured from their centres
(i) If the crystal lattice consists of SCC, the atom which is present at the comers touch each other

(ii) In case of FCC, atom present at the comer and the centre of the face touch each other.
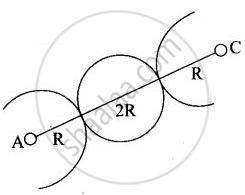
(iii) In case of BCC atom present at the corner and center of the body touch each other
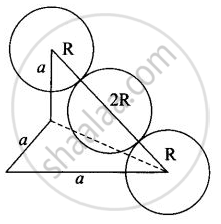
Edge length for different types of unit cells can be tabulated as
| Type of Unit cell | Edge length |
| fcc | `2sqrt(2)r` |
| bcc | `4/sqrt(3)r` |
| scc | 2r |
APPEARS IN
RELATED QUESTIONS
Calculate the efficiency of packing in case of a metal crystal for simple cubic
Packing efficiency for simple cubic structure is ____________.
The correct order of the packing efficiency in different types of unit cells is ______.
Assertion: The total number of atoms present in a simple cubic unit cell is one.
Reason: Simple cubic unit cell has atoms at its corners, each of which is shared between eight adjacent unit cells.
Copper crystallises in a face-centred cubic lattice with unit edge length of 361 pm. What in the radius of copper atom in pm?
The edge length of fee type unit cell of copper having an atomic radius of 127.6 pm is equal to ______
