Advertisements
Advertisements
Question
The efficiency of a heat engine working between the freezing point and boiling point of water is ____________.
Options
6.25%
20%
26.8%
12.5%
Solution
The efficiency of a heat engine working between the freezing point and boiling point of water is 26.8%.
APPEARS IN
RELATED QUESTIONS
Answer in brief:
A gas contained in a cylinder surrounded by a thick layer of insulating material is quickly compressed has work been done?
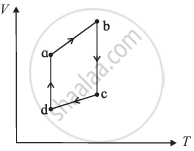
The figure shows the V-T diagram for one cycle of a hypothetical heat engine which uses the ideal gas. Draw the p-V diagram diagram of the system.
State Clausius form of the second law of thermodynamics.
Suppose a person wants to increase the efficiency of the reversible heat engine that is operating between 100°C and 300°C. He had two ways to increase efficiency.
- By decreasing the cold reservoir temperature from 100°C to 50°C and keeping the hot reservoir temperature constant
- by increasing the temperature of the hot reservoir from 300°C to 350°C by keeping the cold reservoir temperature constant.
Which is the suitable method?
For a heat engine operating between temperatures t1 °C and t2 °C, its efficiency will be ______.
A heat engine operates between a cold reservoir at temperature T2 = 400 K and a hot reservoir at temperature T1. It takes 300 J of heat from the hot re ervoir and delivers 240 J of heat to the cold reservoir in a cycle. The minimum temperature of the hot reservoir has to be ______ K.
Which statement is incorrect?
What does a heat engine consist of?
What is a heat engine?
Mention any two elements of a heat engine.
