Advertisements
Advertisements
Question
The melting points and solubility in water of amino acids are generally higher than that of the corresponding halo acids. Explain.
Solution 1
Both acidic (carboxyl) as well as basic (amino) groups are present in the same molecule of amino acids. In aqueous solutions, the carboxyl group can lose a proton and the amino group can accept a proton, thus giving rise to a dipolar ion known as a zwitter ion.
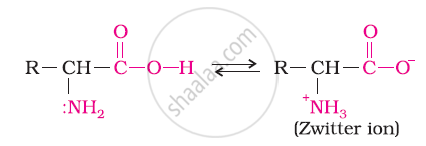
Due to this dipolar behaviour, they have strong electrostatic interactions within them and with water. But halo-acids do not exhibit such dipolar behaviour.
For this reason, the melting points and the solubility of amino acids in water is higher than those of the corresponding halo-acids.
Solution 2
The amino acids exist as zwitter ions, H3N+ — CHR-COO-. Due to this dipolar salt like character, they have strong dipole-dipole attractions. Therefore, their melting points are higher than corresponding haloacids which do not have salt like character.
Due to salt like character, amino acids intereact strongly with water. As a result, their solubility in water is higher than corresponding haloacids which do not have salt like character.
APPEARS IN
RELATED QUESTIONS
Amino acids show amphoteric behaviour. Why?
Define the following terms: Essential amino acids
What are amino acids?
Write the correct reaction for formation of the peptide bond between amino acids
What is the difference between acidic amino acids and basic amino acids?
The number of tripeptides formed by 3 different amino acids.
Which amino acids are chiral?
Match the following:
| I | II |
| (i) Amino acids | (A) protein |
| (ii) Thymine | (B) Nucleic acid |
| (iii) Insulin | (C) DNA |
| (iv) phosphodiester linkage | (D) Zwitter ion |
Which of the following is the best matched options?
Which of the following acids is a vitamin?
Amino acids can be classified as α–, β–, γ–, δ– and so on depending upon the relative position of amino group with respect to carboxyl group. Which type of amino acids form polypetide chain in proteins?
Amino acids behave like salts rather than simple amines or carboxylic acids. Explain.
Describe the term D- and L- configuration used for amino acids with examples.
X – Amino acid behave as crystalline ionic solid and have high melting-point due to the present of:-
Which of the following X – amino acids is not optically active?
Which of the following is a basic amino acid?
Proteins are condensation polymers of:
