Advertisements
Advertisements
Question
The second ionization enthalpies of chromium and manganese are 1592 and 1509 kJ/mol respectively. Explain the lower value of Mn.
Solution
Mn+ has 3d54s1 configuration and configuration of Cr+ is 3d5, therefore, ionisation enthalpy of Mn+ is lower than Cr+.
APPEARS IN
RELATED QUESTIONS
Why do interstitial compounds have higher melting points than corresponding pure metals?
Complete the following chemical equations:
`(i) Cr_2O_7^(2-)+6Fe^(2+)+14H^+ ->`
`(ii) 2CrO_4^(2-)+2H^+ ->`
`(iii) 2MnO_4^-+5C_2O_4^(2-)+16H^+ ->`
Account for the following:
Cu+2 salts are coloured, while Zn2+ salts are white.
Dissociation of H2S is suppressed in acidic medium.
While filling up of electrons in the atomic orbitals, the 4s orbital is filled before the 3d orbital but reverse happens during the ionisation of the atom. Explain why?
On the basis of the figure given below, answer the following questions:
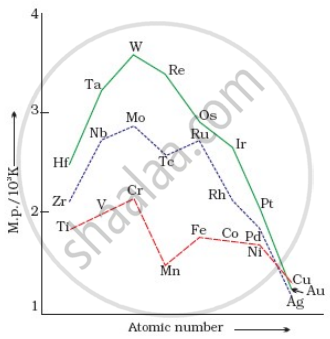
- Why Manganese has lower melting point than Chromium?
- Why do transition metals of 3d series have lower melting points as compared to 4d series?
- In the third transition series, identify and name the metal with the highest melting point.
Which does not belong to first transition series?
Which of the following maxm magnetic moment?
The disproportionation of \[\ce{MnO^{2-}_4}\] in acidic medium resulted in the formation of two manganese compounds A and B. If the oxidation state of Mn in B is smaller than that of A, then the spin-only magnetic moment (µ) value of B in BM is ______. (Nearest integer)
Give two similarities in the properties of Sc and Zn.
