Advertisements
Advertisements
Question
The total number of tetrahedral voids in the face-centered unit cell is ______.
Options
6
8
10
12
Solution
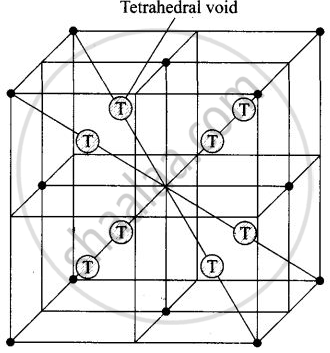
The total number of tetrahedral voids in the face-centered unit cell is 8.
Explanation:
Fee unit cell contains 8 tetrahedral voids at centre of each 8 smaller cube of a unit cell as shown below
Eight tetrahedral voids per fee unit vell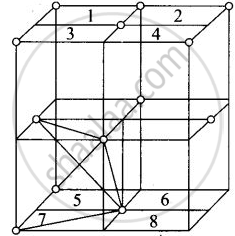
Each cube represented by numeric 1, 2, 3, 4, 5, 6, 7, 8 contains one tetrahedral void.
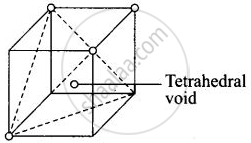
Each cube contains one tetrahedral void its body centre as shown above
APPEARS IN
RELATED QUESTIONS
Which crystal system has axial angles equal to 90°?
Which crystal system has a ≠ b ≠ c?
How many lithium atoms are present in a unit cell with edge length 3.5 Å and density 0.53 g cm−3? (Atomic mass of Li = 6.94):
The distance between Na– and Cl– ions in NaCl with a density 2.165 g cm–3 is __________.
Assertion: Total number of octahedral voids present in unit cell of cubic close packing including the one that is present at the body centre, is four.
Reason: Besides the body centre there is one octahedral void present at the centre of each of the six faces of the unit cell and each of which is shared between two adjacent unit cells.
Sodium crystallises in a body-centred cubic unit cell. (bcc) with edge length 4.29 A. what is the radius of the sodium atom? What is the length of the body diagonal of the unit cell?
The density of a crystal is given by the formula
The correct option for the number of body-centered unit cells in all 14 types of Bravais lattice unit cells is ______.
A certain element crystallises in a bcc lattice of unit cell edge length 27 A°. If the same element under the same conditions crystallises in the fcc lattice, the edge length of the unit cell in A° will be ______. (Round off to the Nearest Integer).
[Assume each lattice point has a single atom]
Gold crystallizes in a face-centered cubic lattice. If the length of the edge of the unit cell is 407 pm. The density of gold assuming it to be spherical is ______ g/cm3. Atomic mass of gold = 197 amu.
