Advertisements
Advertisements
Question
What is the action of heat on potassium permanganate?
Solution
Action of heat on KMnO4:
KMnO4 when heated to 473 K, readily decomposes giving oxygen.
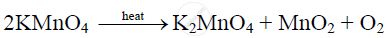
At red heat, potassium permanganate decomposes into potassium manganate (K2MnO3) and oxygen.
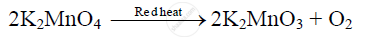
APPEARS IN
RELATED QUESTIONS
Absolute entropies of solids, liquids and gases can be determined by
- Measuring heat capacity of substance at various temperatures
- Subtracting standard entropy of reactants from products
- Measuring vibrational motion of molecules
- Using formula ΔS° = ST° - SO°
Determine whether the reactions with the following ΔH and ΔS values are spontaneous or non-spontaneous. State whether the reactions are exothermic or endothermic.
(a) ΔH = -110kJ, ΔS = + 40JK-1 at 400 K
(b) ΔH = + 40kJ, ΔS = -120JK-1 at 250K
300 M mol of perfect gas occupies 13 L at 320 K. Calculate the work done in joules when the gas expands-
(a) isothermally against a Constant external pressure of 0.20atm.
(b) isothermal and reversible process.
(c) into vaccum until the volume of gas is increased by 3L (R=8.314J mol-1 K-1)
One mole of a gas expands by 3L against a constant pressure of 3 atmosphere. Calculate the work done in -
(a) L.atmosphere
(b) Joules
(c) Calories
Which of the following pairs is an intensive property?
(A) Density, viscosity
(B) Surface tension, mass
(C) Viscosity, internal energy
(D) Heat capacity, volume
55 L atm of work is obtained when 1.0 mole of an ideal gas is compressed isothermally from
a volume of 28.5 L to 18.5 L, the constant external pressure is
(A) 5.05 atm
(B) 5.5 atm
(C) 0.05 atm
(D) 0.55 atm
For a certain reaction, ∆H = − 50 kJ and ∆S = − 80 J K-1, at what temperature does the
reaction turn from spontaneous to non-spontaneous?
(A) 6.25 K
(B) 62.5 K
(C) 625 K
(D) 6250 K
Derive the equation : W = - PextAV
5 moles of helium expand isothermally and reversibly from a pressure 40 × 10-5 N m-2 to 4 × 10-5 N m-2 at 300 K. Calculate the work done, change in internal energy and heat absorbed during the expansion. (R = 8.314 J K-1 mol-1).
