Advertisements
Advertisements
Question
What are cathode rays? How are these rays formed?
Solution
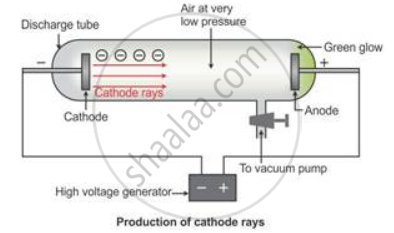
Cathode rays are the beam of electrons which travel from the negatively charged end (i.e. cathode to anode) of a vacuum tube, across a voltage difference between the electrodes placed at each end.
Formation of cathode rays:
Generally gases are poor conductors of electricity. However, when a high voltage charge from an induction coil is applied to tubes filled with gases at very low pressure (0.01 mm of mercury), the gases become good conductors of electricity and begin to flow in the form of rays.
These rays are called cathode rays and they travel from the cathode towards the anode.
APPEARS IN
RELATED QUESTIONS
Name the subatomic particle whose relative charge is : –1
Fill in the blanks of the following statements :
An atom has atomic mass number 23 and atomic number 11. The atom has_________ electrons.
Cathode rays are a beam of fast moving _________
The maximum number of electrons that can be accommodated in L shell are_________
The maximum number of electrons that can go into the M shell is _________
What is the nature of the charge on Cathode rays .
What are valence electrons?
An element A has 1 electron in its first shell. It combines with element B having 7 electrons in its third shell. What type of bond is formed?
What is the term defined below?
A bond formed by a shared pair of electrons, each bonding atom contributing one electron to the pair.
Differentiate between the following term:
Electron and proton
Cathode rays are made up of
Atoms of all metals will have ______ electrons in their outermost orbit.
Assertion: Helium has four electrons in the outermost orbit.
Reason: Neon has eight electrons in the outermost orbit.
