Advertisements
Advertisements
Question
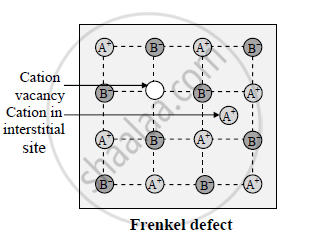
What are Frenkel defect
Solution
Frenkel defect:
1) When cation or anion from ionic solid leaves its regular site and moves to occupy a place between the lattice site called interstitial position, the defect is called interstitial defect or Frenkel defect.
2)The presence of this defect does not alter the density of the solid.
3) This defect is common when the difference in ionic radii of cations and anions is large
4) This defect is observed in AgCl solid because of Ag+ ions or ZnS solid because
of Zn++ ions.
APPEARS IN
RELATED QUESTIONS
Explain impurity defect in stainless steel with diagram
What type of point defect is produced when AgCl is doped with CdCl2?
Define the following term
Schottky defect
What type of non-stoichiometric point defect is responsible for the pink colour of LiCl ?
What type of stoichiometric defect is shown by NaCl ?
Which stoichiometric defect does not change the density of the crystal?
Which stoichiometric defect decreases the density of the crystal?
What type of defect can arise when a solid is heated? Which physical property is affected by it and in what way?
What type of stoichiometric defect is shown by:
(i) ZnS
(ii) AgBr
Schottky defects are observed in which solid among the following?
(a) Brass
(b) Cesium Chloride
(c) Zinc sulphide
(d) Stainless steel
The defects which arise due to irregularities or deviations from the ideal arrangement in an entire row of lattice points are called ______
