Advertisements
Advertisements
Questions
Define the following term
Schottky defect
Explain the following terms with suitable examples
Schottky defect
What are Schottky defect
Solution 1
Schottky defect is defined as the one in which equal number of cations and anions are missing from their lattice positions in an ionic compound.
Solution 2
Schottky defect: Schottky defect is basically a vacancy defect shown by ionic solids. In this defect, an equal number of cations and anions are missing to maintain electrical neutrality. It decreases the density of a substance. Significant number of Schottky defects is present in ionic solids. For example, in NaCl, there are approximately 106 Schottky pairs per cm3 at room temperature. Ionic substances containing similar-sized cations and anions show this type of defect. For example: NaCl, KCl, CsCl, AgBr, etc.
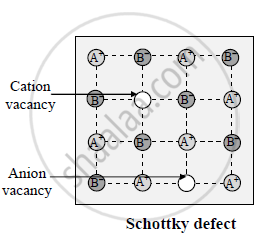
Solution 3
Schottky defect:
1) Sometimes during crystallisation, some of the places of the constituent particles
remain unoccupied and the defect generated is called vacancy defect or Schottky defect
2) The unoccupied positions are called vacancies
3) It results in the decrease in density of the substance.
4) In case of ionic solids, cations and anions in stoichiometric proportions remain absent from their position to maintain electrical neutrality.
5) In Ionic compounds, this defect is known as Schottky defect
6) The defects are observed in solids with cations and anions having almost equal size like NaCl, KCl, CsCl, etc.
APPEARS IN
RELATED QUESTIONS
Explain impurity defect in stainless steel with diagram
What type of point defect is produced when AgCl is doped with CdCl2?
What type of non-stoichiometric point defect is responsible for the pink colour of LiCl ?
What type of stoichiometric defect is shown by NaCl ?
Which stoichiometric defect does not change the density of the crystal?
Which stoichiometric defect decreases the density of the crystal?
What type of defect can arise when a solid is heated? Which physical property is affected by it and in what way?
What type of stoichiometric defect is shown by:
(i) ZnS
(ii) AgBr
Schottky defects are observed in which solid among the following?
(a) Brass
(b) Cesium Chloride
(c) Zinc sulphide
(d) Stainless steel
What are Frenkel defect
The defects which arise due to irregularities or deviations from the ideal arrangement in an entire row of lattice points are called ______
