Science (English Medium)
Academic Year: 2014-2015
Date: March 2015
Advertisements
Account for the following:
Cu+2 salts are coloured, while Zn2+ salts are white.
Chapter: [0.04] d-block and f-block Elements
Which would undergo SN1 reaction faster in the following pair and why?
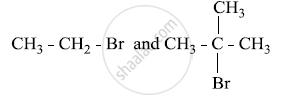
Chapter: [0.06] Haloalkanes and Haloarenes
How much charge is required for the reduction of 1 mol of Zn2+ to Zn?
Chapter: [0.02] Electrochemistry
Write the dispersed phase and dispersion medium of butter.
Chapter: [0.05] Surface Chemistry
Write the IUPAC name of the given compound:
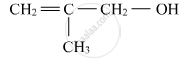
Chapter: [0.07] Alcohols, Phenols and Ethers
Write the structures of the following molecules: H2SO3
Chapter: [0.07] P - Block Elements
Write the structures of the following molecules: XeOF4
Chapter: [0.07] P - Block Elements
Write down the IUPAC name of the complex [Pt(en)2Cl2]2+. What type of isomerism is shown by this complex?
Chapter: [0.05] Coordination Compounds
Using IUPAC norms write the formulate for the following coordination compounds : Hexaamminecobalt (III) chloride
Chapter: [0.05] Coordination Compounds
Using IUPAC norms write the formulate for the following coordination compounds :
Potassium tetrachloridonickelate (II)
Chapter: [0.05] Coordination Compounds
Write two factors that affect the rate of reaction.
Chapter: [0.03] Chemical Kinetics
Arrange the following in increasing order of their basic strength :
C6H5 – NH2, C6H5 – CH2 – NH2, C6H5 – NH – CH3
Chapter: [0.09] Amines
Arrange the following in increasing order of their basic strength :

Chapter: [0.09] Amines
Why does a solution containing non-volatile solute have higher boiling point than the pure solvent ?
Chapter: [0.01] Solutions
Why is elevation of boiling point a colligative property?
Chapter: [0.01] Solutions
What is the principle behind the zone refining of metals?
Chapter: [0.06] General Principles and Processes of Isolation of Elements
What is the role of silica in the extraction of copper?
Chapter: [0.06] General Principles and Processes of Isolation of Elements
How is 'cast iron' different from 'pig iron'?
Chapter: [0.06] General Principles and Processes of Isolation of Elements
Account for the following:
N2 is less reactive at room temperature.
Chapter: [0.07] P - Block Elements
Give reasons for the following : H2Te is the strongest reducing agent amongst all the hydrides of Group 16 elements.
Chapter: [0.07] P - Block Elements
Account for the following:
Helium is used in diving apparatus.
Chapter: [0.07] P - Block Elements
Write the hybridization and shape of the following complexe : [CoF6]3–
(Atomic number : Co = 27, Ni = 28)
Chapter: [0.05] Coordination Compounds
Write the hybridization and shape of the following complexe : [Ni(CN)4]2–
(Atomic number : Co = 27, Ni = 28)
Chapter: [0.05] Coordination Compounds
Out of NH3 and CO, which ligand forms a more stable complex with a transition metal and why?
Chapter: [0.05] Coordination Compounds
Advertisements
How do you convert the following: C6H5CONH2 to C6H5NH2
Chapter: [0.09] Amines
How do you convert the following:
Aniline to phenol
Chapter: [0.07] Alcohols, Phenols and Ethers
How do you convert the following: Ethanenitrile to ethanamine
Chapter: [0.09] Amines
Write the chemical equations involved when aniline is treated with the following reagents:
Br2 water
Chapter: [0.09] Amines
Write the chemical equations involved when aniline is treated with the following reagents:
CHCI3 + KOH
Chapter: [0.09] Amines
Write the chemical equations involved when aniline is treated with the following reagents: HCI
Chapter: [0.09] Amines
Write the names and structure of the monomers of the following polymers: Buna − S
Chapter:
Write the names and structures of the monomers of the following polymers: Glyptal
Chapter:
Write the names and structures of the monomers of the following polymers: Polyvinyl chloride
Chapter: [0.15] Polymers
Write the product obtained when D-glucose reacts with H2N − OH.
Chapter: [0.1] Biomolecules
Amino acids show amphoteric behaviour. Why?
Chapter: [0.1] Biomolecules
Why Vitamin C cannot be stored in our body ?
Chapter: [0.1] Biomolecules
Calculate the freezing point of the solution when 31 g of ethylene glycol (C2H6O2) is dissolved in 500 g of water.
(Kf for water = 1.86 K kg mol–1)
Chapter: [0.01] Solutions
Define the following term: Primitive unit cells
Chapter: [0.01] Solid State
Define the following term
Schottky defect
Chapter: [0.01] Solid State
Define the following term: Ferromagnetism
Chapter: [0.01] Solid State
Write the structure of the major product in each of the following reaction :
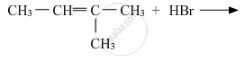
Chapter:
Write the structure of the major product in each of the following reaction :
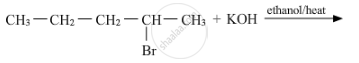
Chapter:
Write the structure of the major product in each of the following reaction :
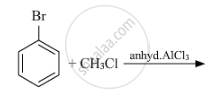
Chapter: [0.06] Haloalkanes and Haloarenes
Give reasons for the following : Phenol is more acidic than ethanol.
Chapter: [0.07] Alcohols, Phenols and Ethers
Give reasons for the following : Boiling point of ethanol is higher in comparison to methoxymethane.
Chapter: [0.07] Alcohols, Phenols and Ethers
Give reasons for the following:
(CH3)3C–O–CH3 on reaction with HI gives (CH3)3C–I and CH3–OH as the main products and not (CH3)3C–OH and CH3–I.
Chapter: [0.06] Haloalkanes and Haloarenes
The rate constant of a first order reaction increases from 2 × 10−2 to 4 × 10−2 when the temperature changes from 300 K to 310 K. Calculate the energy of activation (Ea).
(log 2 = 0.301, log 3 = 0.4771, log 4 = 0.6021)
Chapter: [0.03] Chemical Kinetics
Define the following term : Brownian movement
Chapter: [0.05] Surface Chemistry
Advertisements
Define the following term :
Peptization
Chapter: [0.1] Biomolecules
Define the following term : Multimolecular colloids
Chapter: [0.05] Surface Chemistry
Seeing the growing cases of diabetes and depression among young children, Mr Lugani, the principal of one reputed school, organised a seminar in which he invites parents and principals. They all resolved this issue by strictly banning junk food in schools and introducing healthy snacks and drinks like soup, lassi, milk etc. in school canteens. They also decided to make compulsory half an hour of daily physical activities for the students in the health survey in most of the school and discovered a tremendous improvement in the health of the students
After reading the above passage, answer the following questions:
(i) What are the values (at least two) displayed by Mr Lugani?
(ii) As a student, how can you spread awareness about this issue?
(iii) What are antidepressant drugs? Give an example.
(iv) Name the sweetening agent used in the preparation of sweets for a diabetic patient.
Chapter: [0.16] Chemistry in Everyday Life
Calculate e.m.f. and ∆G for the following cell:
Mg (s) |Mg2+ (0.001M) || Cu2+ (0.0001M) | Cu (s)
`"Given :" E_((Mg^(2+)"/"Mg))^0=−2.37 V, E_((Cu^(2+)"/"Cu))^0=+0.34 V.`
Chapter: [0.02] Electrochemistry
The conductivity of 0.20 mol L−1 solution of KCl is 2.48 × 10−2 S cm−1. Calculate its molar conductivity and degree of dissociation (α). Given λ0 (K+) = 73.5 S cm2 mol−1 and λ0 (C1−) = 76.5 S cm2 mol−1.
Chapter: [0.02] Electrochemistry
What type of battery is mercury cell? Why is it more advantageous than dry cell?
Chapter: [0.02] Electrochemistry
Account for the following :
Zr and Hf have almost similar atomic radii.
Chapter: [0.04] d-block and f-block Elements
Give reasons: Transition metals show variable oxidation states.
Chapter: [0.04] d-block and f-block Elements
Account for the following:
Cu+ ion is unstable in aqueous solution.
Chapter: [0.04] d-block and f-block Elements
Complete the following equations : 2 MnO2 + 4 KOH + O2 →
Chapter: [0.04] d-block and f-block Elements
Complete the following equations : 2 Na2CrO4 + 2 H + →
Chapter: [0.04] d-block and f-block Elements
|
`E_((M^(2+)/M)` |
Cr | Mn | Fe | Co | Ni | Cu |
| -0.91 | -1.18 | -0.44 | -0.28 | -0.25 | -0.34 |
From the given data of E0 values, answer the following questions :
(1) Why is `E_(((Cu^(2+))/(Cu)))` value exceptionally positive
(2) Why is `E_(((Mn^(2+))/(Mn)))` value is highly negative as compared to other elements
(3) Which is the stronger reducing agents Cr2+ or Fe2+ ? Give Reason.
Chapter: [0.04] d-block and f-block Elements
Why do actinoids show a wide range of oxidation states?
Chapter: [0.04] d-block and f-block Elements
Write on similarity between the chemistry of lanthanoids and actinoids.
Chapter: [0.04] d-block and f-block Elements
A compound 'A' of molecular formula C2H3OCl undergoes a series of reactions as shown below. Write the structures of A, B, C and D in the following reactions :
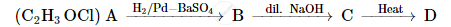
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Distinguish between:
C6H5-COCH3 and C6H5-CHO
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Distinguish between the following : Benzoic acid and methyl benzoate
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the structure of 2-methylbutanal.
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the structures of the main products when acetone (CH3 − CO − CH3) reacts with the following reagent :
Zn − Hg/conc. HCl
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the structures of the main products when acetone (CH3 − CO − CH3) reacts with the following reagent :
H2N − NHCONH2/H+
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Write the structures of the main products when acetone (CH3 − CO − CH3) reacts with the following reagents :
CH3MgBr and then H3O+
Chapter: [0.07] Alcohols, Phenols and Ethers
Arrange the following in the increasing order of their boiling points : C2H5OH, CH3 − CHO, CH3 − COOH
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Give a simple chemical test to distinguish between the following pair of compounds :
CH3CH2CHO and CH3CH2COCH3
Chapter: [0.08] Aldehydes, Ketones and Carboxylic Acids
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 12 Chemistry with solutions 2014 - 2015
Previous year Question paper for CBSE Class 12 -2015 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Chemistry, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 12.
How CBSE Class 12 Question Paper solutions Help Students ?
• Question paper solutions for Chemistry will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
