Advertisements
Advertisements
Question
What are hydrocarbons? Give one example.
Solution
Organic compounds containing hydrogen along with carbon are known as hydrocarbons.
Example: Ethene, Methane
APPEARS IN
RELATED QUESTIONS
How would you distinguish experimentally between an alcohol and a carboxylic acid?
Organic compounds having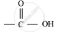 functional group are known as
functional group are known as
Give one exampleof the compound having the following functional group:
Alcohol group
Draw the structures for the following compounds of Pentanal
What happens when ethanol is heated with concentrated sulphuric acid at 170°C? Write the equation of the reaction which takes place.
Identify the functional group of the HCHO ?
What is meant by functional group in carbon compounds? Write in tabular form the structural formula and the functional group present in the following compounds:
(i) Ethanol
(ii) Ethanoic acid
Name the oxidising agent used for the conversion of ethanol to ethanoic acid. Distinguish between ethanol and ethanoic acid on the basis of (i) litmus test, (ii) reaction with sodium carbonate
Ethane, Ethene, Ethanoic acid, Ethyne, Ethanol
From the compounds given above, name
The compound with -OH as apart of its structure.
Choose the correct word/phrase from the options given below to complete the following sentence:
The product formed when ethene gas reacts with water in the presence of sulphuric acid is ______.
Find the odd one out and explain:
Draw the structures for ethanol.
Write the characteristics of ethanol.
Rectified spirit is an aqueous solution that contains about ______ of ethanol.
A gas is evolved when ethanol reacts with sodium. Name the gas evolved and also write the balanced chemical equation of the reaction involved.
How would you bring about the following conversions? Name the process and write the reaction involved.
Name the compound formed when ethanol is heated at 443 K in the presence of conc. \[\ce{H2SO4}\] and draw its electron dot structure.
State the role of conc. \[\ce{H2SO4}\] in the reaction.
Give the balanced chemical equation of the following reaction:
Oxidation of ethanol by acidified potassium dichromate.
Give the balanced chemical equation of the following reaction:
Oxidation of ethanol by acidified potassium dichromate.
Give the balanced chemical equation of the following reaction:
Combustion of ethanol.
