Advertisements
Advertisements
Question
What are the necessary conditions for any system to be aromatic?
Answer in Brief
Solution 1
A compound is said to be aromatic if it satisfies the following three conditions:
- It should have a planar structure.
- The π–electrons of the compound are completely delocalized in the ring.
- The total number of π–electrons present in the ring should be equal to (4n + 2), where n = 0, 1, 2 … etc. This is known as Huckel’s rule.
shaalaa.com
Solution 2
The necessary conditions for a molecule to be aromatic are:
- It should have a single cyclic cloud of delocalised n-electrons above and below the plane of the molecule.
- It should be planar. This is because complete delocalization of n-electrons is possible only if the ring is planar to allow cyclic overlap of p-orbitals.
- It should contain Huckel number of electrons, i.e., (4n + 2) n-electrons where n = 0, 1, 2, 3 etc.
A molecule which does not satisfy any one or more of the above conditions is said to be non-aromatic.
shaalaa.com
Aromatic Hydrocarbons - Aromaticity (Huckel Rule)
Is there an error in this question or solution?
Chapter 13: Hydrocarbons - EXERCISES [Page 405]
APPEARS IN
RELATED QUESTIONS
Explain why the following system is not aromatic?

Explain why the following system is not aromatic?
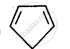
Explain why the following system is not aromatic?
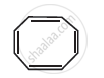
Four structures are given in options (i) to (iv). Examine them and select the aromatic structures.
| (i) | 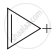 |
| (ii) | 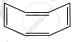 |
| (iii) | 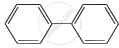 |
| (iv) | 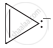 |
Cyclopentadienyl anion is ______.
Among the following, the aromatic compounds are:
| (A) | 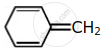 |
| (B) |  |
| (C) | 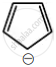 |
| (D) | 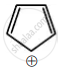 |
Choose the correct answer from the following options:
