Advertisements
Advertisements
Question
Solution
A calorimeter is a device used to measure the quantity of heat transferred to or from an object.
It is made of copper because:
i. Copper is a good conductor of heat so it attains the temperature of its contents in a very short time.
ii. It has low specific heat (390 Jkg-1K-1). Therefore, it will take only a very little part of the heat energy given out in the experiment.
APPEARS IN
RELATED QUESTIONS
How can a temperature in degree Celsius be converted into S.I. unit of temperature?
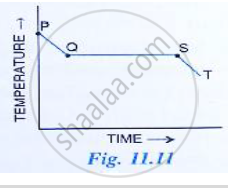
Fig 11. 11 shows the variation in temperature with time when some wax cools from the liquid phase to the solid phase.
(i) In which part of the curve, the wax is in liquid phase?
(ii) What does the part QS of the curve represent?
(iii) In which part of the curve, the wax will be the in the liquid as well as solid phase?
(iv) In which part of the curve, the wax is in solid phase?
- Define Calorimetry.
- Name the material used for making a Calorimeter.
- Why is a Calorimeter made up of thin sheets of the above material answered in ii.
In an experiment. 17 g of ice is used to bring down the temp. of 40 g of water from 34°C to its freezing point. The sp. heat capacity of water is 4.25 J/g°C. Calculate sp. latent heat of ice.
Name two factors on which the heat energy librated by a body on cooling depends.
A mass of 40g of brass of specific heat capacity 0.85 Jg-1 K-1 is heated in an oven and then quickly transferred into 240g of water at 30°C in a calorimeter of mass 60g and specific heat capacity 0.4 Jg-1 K-1. If the final temperature is 50°C. What was the temperature of the oven?
40g of ice at 0°C is used to bring down the temperature of a certain mass of water at 60°C to 10°C. Find the mass of water used.
[Specific heat capacity of water = 4200 J kg-1 °C-1]
[Specific latent heat of fusion of ice = 336 × 103 J kg-1]
Find the final temperature when a mass of 80g of water at 100°C is mixed with a mass of 40g of water at 25°C.
