Advertisements
Advertisements
Question
Solution
(i) Given: Heat Capacity, me = 966 J/°C
Heat energy required, Q = m x C x change in temperature
= 966 x 15 = 14490 J
(ii) Q = m x C x T
Specific heat capacity, C = `"Q"/("m"Δ "T")`
= `14490/(2 xx 15)`
= 483 Jkg-1 °C-1
APPEARS IN
RELATED QUESTIONS
(a) Calculate the heat capacity of a copper vessel of mass 150g if the specific heat capacity of copper is `410 J kg^-1 K^-1`
(b) How much heat energy will be required to increase the temperature of the vessel in part (a) from 25°C to 35°C?
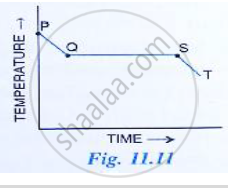
Fig 11. 11 shows the variation in temperature with time when some wax cools from the liquid phase to the solid phase.
(i) In which part of the curve, the wax is in liquid phase?
(ii) What does the part QS of the curve represent?
(iii) In which part of the curve, the wax will be the in the liquid as well as solid phase?
(iv) In which part of the curve, the wax is in solid phase?
Write the SI unit of:
(i) Amount of heat
(ii) Heat capacity
(iii) Specific Heat capacity
In an experiment. 17 g of ice is used to bring down the temp. of 40 g of water from 34°C to its freezing point. The sp. heat capacity of water is 4.25 J/g°C. Calculate sp. latent heat of ice.
Name the material of which it is made of. Give two reasons for using the material stated by you.
Conduction is the way of heat transfer which takes place in a ______.
The process of converting a substance from solid state to gaseous state is called condensation.
The device which is used to measure the heat capacity of the liquid is ______.
