Advertisements
Advertisements
Question
What do you mean by electron emission? Explain briefly various methods of electron emission.
Solution
Electron emission:
- Free electrons possess some kinetic energy and this energy is different for different electrons. The kinetic energy of the free electrons is not sufficient to overcome the surface barrier.
- Whenever additional energy is given to the free electrons, they will have sufficient energy to cross the surface barrier. And they escape from the metallic surface.
-
The liberation of electrons from any surface of a substance is called electron emission.
There are mainly four types of electron emission which are given below.
(i) Thermionic emission: When a metal is heated to a high temperature, the free electrons on the surface of the metal get sufficient energy in the form of thermal energy so that they are emitted from the metallic surface. This type of emission is known as thermionic emission.
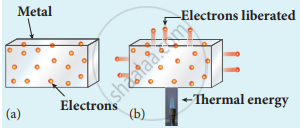
Electrons in the (a) metal (b) heated metal
The intensity of the thermionic emission (the number of electrons emitted) depends on the metal used and its temperature.

Thermionic emission from hot filament of cathode ray tube or x-ray tube
Examples: cathode ray tubes, electron microscopes, X-ray tubes, etc.
(ii) Field emission: Electric field emission occurs when a very strong electric field is applied across the metal. This strong field pulls the free electrons and helps them to overcome the surface barrier of the metal.

Field emission
Examples: Field emission scanning electron microscopes, Field-emission display, etc.
(iii) Photo electric emission: When electromagnetic radiation of suitable frequency is incident on the surface of the metal, the energy is transferred from the radiation to the free electrons. Hence, the free electrons get sufficient energy to cross the surface barrier and the photo electric emission takes place. The number of electrons emitted depends on the intensity of the incident radiation.

Photo electric emission
Examples: Photo diodes, photo electric cells, etc.
(iv) Secondary emission: When a beam of fast-moving electrons strikes the surface of the metal, the kinetic energy of the striking electrons is transferred to the free electrons on the metal surface. Thus the free electrons get sufficient kinetic energy so that the secondary emission of, electron occurs.
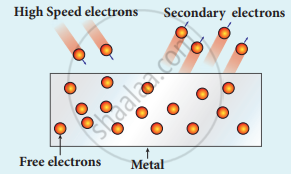
Secondary emission of electrons
Examples: Image intensifies, photo multiplier tubes, etc.
APPEARS IN
RELATED QUESTIONS
Find the maximum frequency of X-rays produced by 30 kV electrons.
How does one explain the emission of electrons from a photosensitive surface with the help of Einstein's photoelectric equation?
A diode value is connected to a battery and a load resistance. The filament is heated, so that a constant current is obtained in the circuit. As the cathode continuously emits electrons, does it become more and more positively charged?
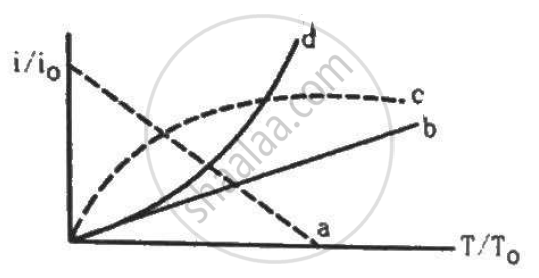
Let i0 be the thermionic current from a metal surface when the absolute temperature of the surface is T0. The temperature is slowly increased and the thermionic current is measured as a function of temperature. Which of the following plots may represent the variation in (i/i0) against (T/T0)?
The constant A in the Richardson−Dushman equation for tungsten is 60 × 104 A m−2K−2. The work function of tungsten is 4.5 eV. A tungsten cathode with a surface area 2.0 × 10−5 m2 is heated by a 24 W electric heater. In steady state, the heat radiated by the heater and the cathode equals the energy input by the heater and the temperature becomes constant. Assuming that the cathode radiates like a blackbody, calculate the saturation current due to thermions. Take Stefan's Constant = 6 × 10−8 W m−2 K−1. Assume that the thermions take only a small fraction of the heat supplied.
Emission of electrons by the absorption of heat energy is called ____________ emission.
Define the work function of a metal. Give its unit.
A 150 W lamp emits light of the mean wavelength of 5500 Å. If the efficiency is 12%, find out the number of photons emitted by the lamp in one second.
In which case is electron emission from a metal not known?
Photoelectric emission is observed from a metallic surface for frequencies ν1 and ν2 of the incident light (ν1 > ν2). If the maximum value of kinetic energy of the photoelectrons emitted in the two cases are in the ration 1 : n then the threshold frequency of the metallic surface is ______.
