Advertisements
Advertisements
Question
What do you understand by the invisible spectrum?
Solution
The part of spectrum beyond the red and the violet ends is called the invisible spectrum as our eyes do not respond to the spectrum beyond the red and the violet extremes.
APPEARS IN
RELATED QUESTIONS
If the earth did not have an atmosphere, would its average surface temperature be higher or lower than what it is now?
Name three properties of ultraviolet radiations which are similar to visible light.
The wavelength of X-rays is 0.01 Å. Calculate its frequency. State the assumption made, if any.
If the potential difference applied to the tube is doubled and the separation between the filament and the target is also doubled, the cutoff wavelength
The Kβ X-rays from certain elements are given below. Draw a Moseley-type plot of √v versus Z for Kβ radiation.
| Element | Ne | P | Ca | Mn | Zn | Br |
| Energy (keV) | 0.858 | 2.14 | 4.02 | 6.51 | 9.57 | 13.3 |
All components of the electromagnetic spectrum in a vacuum have the same ______
Identify the electromagnetic wave whose wavelength range is from about 10-12 m to about 10-8 m. Write one use of this.
Below is an incomplete table showing the arrangement of electromagnetic spectrum in the increasing order of their wavelength. Complete the table:
| Gamma ray | X - ray | UV rays | Visible rays | Infrared | A | Radio waves |
- Identify the radiation A.
- Name the radiation used to detect fracture in bones.
- Name one property common to both A and Radio waves.
What happens when an electron collides with a positron?
In an atom X, electrons absorb the energy from an external source. This energy “excites” the electrons from a lower-energy level to a higher-energy level around the nucleus of the atom. When electrons return to the ground state, they emit photons.
The figure below is the energy level diagram of atom X with three energy levels, E1 = 0.00eV, E2 = 1.78eV and E3 = 2.95eV. The ground state is considered 0 eV for reference. The transition of electrons takes place between levels E1 and E2.
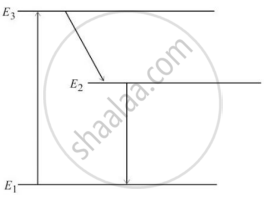
- What wavelength of radiation is needed to excite the atom to energy level E2 from E1?
- Suppose the external source has a power of 100 W. What would be the rate of photon emission?
