Advertisements
Advertisements
Question
What is the maximum number of emission lines when the excited electron of an H atom in n = 6 drops to the ground state?
Solution
When the excited electron of an H atom in n = 6 drops to the ground state, the following transitions are possible:
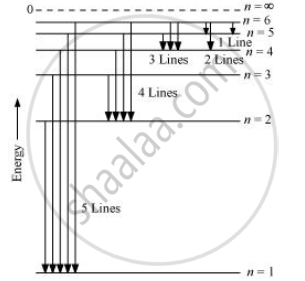
Hence, a total number of (5 + 4 + 3 + 2 + 1) 15 lines will be obtained in the emission spectrum.
The number of spectral lines produced when an electron in the nth level drops down to the ground state is given by `("n"("n"-1))/2`
Given, n = 6
Number of spectral lines =` (6(6-1))/2 = 15`
APPEARS IN
RELATED QUESTIONS
What is the energy in joules, required to shift the electron of the hydrogen atom from the first Bohr orbit to the fifth Bohr orbit and what is the wavelength of the light emitted when the electron returns to the ground state? The ground state electron energy is –2.18 × 10–11 ergs.
The electron in hydrogen atom is initially in the third excited state. What is the maximum number of spectral lines which can be emitted when it finally moves to the ground state?
Which of the following parameters are the same for all hydrogen-like atoms and ions in their ground states?
According to Maxwell's theory of electrodynamics, an electron going in a circle should emit radiation of frequency equal to its frequency of revolution. What should be the wavelength of the radiation emitted by a hydrogen atom in ground state if this rule is followed?
A beam of light having wavelengths distributed uniformly between 450 nm to 550 nm passes through a sample of hydrogen gas. Which wavelength will have the least intensity in the transmitted beam?
The light emitted in the transition n = 3 to n = 2 in hydrogen is called Hα light. Find the maximum work function a metal can have so that Hα light can emit photoelectrons from it.
Consider a neutron and an electron bound to each other due to gravitational force. Assuming Bohr's quantization rule for angular momentum to be valid in this case, derive an expression for the energy of the neutron-electron system.
State any two Bohr’s postulates and write the energy value of the ground state of the hydrogen atom.
If l3 and l2 represent angular momenta of an orbiting electron in III and II Bohr orbits respectively, then l3: l2 is :
The dissociation constant of a weak base (BOH) is 1.8 × 10−5. Its degree of dissociation in 0.001 M solution is ____________.
According to Bohr's theory, an electron can move only in those orbits for which its angular momentum is integral multiple of ____________.
Using Bohr's postulates derive the expression for the radius of nth orbit of the electron.
For the ground state, the electron in the H-atom has an angular momentum = h, according to the simple Bohr model. Angular momentum is a vector and hence there will be infinitely many orbits with the vector pointing in all possible directions. In actuality, this is not true ______.
When an electron falls from a higher energy to a lower energy level, the difference in the energies appears in the form of electromagnetic radiation. Why cannot it be emitted as other forms of energy?
The number of times larger the spacing between the energy levels with n = 3 and n = 8 spacing between the energy level with n = 8 and n = 9 for the hydrogen atom is ______.
Find the ratio of energies of photons produced due to transition of an election of hydrogen atom from its (i) second permitted energy level to the first level. and (ii) the highest permitted energy level to the first permitted level.
What is the energy associated with first orbit of Li2+ (RH = 2.18 × 10-18)?
A 20% efficient bulb emits light of wavelength 4000 Å. If the power of the bulb is 1 W, the number of photons emitted per second is ______.
[Take, h = 6.6 × 10-34 J-s]
State three postulates of Bohr's theory of hydrogen atom.
How much is the angular momentum of an electron when it is orbiting in the second Bohr orbit of hydrogen atom?
