Advertisements
Advertisements
Question
What is the structural difference between HDP and LDP? How does the structure account for different behaviour and nature, hence the use of a polymer?
Solution
Low-density polythene: It is obtained by the polymerisation of ethene under high pressure of 1000 to 2000 atmospheres at a temperature of 350 K to 570 K in the presence of traces of dioxygen or a peroxide initiator (catalyst). The low-density polythene (LDP) is obtained through the free radical addition and H-atom abstraction has highly branched structure.
High-density polythene: It is formed when addition polymerisation of ethene takes place in a hydrocarbon solvent in the presence of a catalyst such as triethylaluminium and titanium tetrachloride (Ziegler-Natta catalyst) at a temperature of 333 K to 343 K and under a pressure of 6-7 atmospheres. High-density polythene (HDP) thus produced, consists of linear molecules as shown below and has a high density due to close packing. It is also chemically inert and more tougher and harder. It is used for manufacturing buckets, dustbins, bottles, pipes, etc.
APPEARS IN
RELATED QUESTIONS
Write the names and structures of the monomers of the following polymers: Polyvinyl chloride
Write the name of monomers used for getting the following polymers : Bakelite
Write the names of monomers used for getting the following polymers:
Teflon
Identify the monomer in the following polymeric structures.
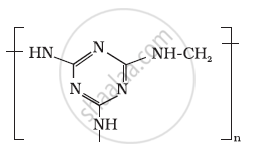
Write the names and structures of the monomers of the following polymers:
(i) Terylene
(ii) Bakelite
(iii) Buna-S
Identify the type of polymer given in the following figure.

To have practical applications why are cross links required in rubber?
Assertion: Network polymers are thermosetting.
Reason: Network polymers have high molecular mass.
Which of the following is a basic dye?
Which one is disperse dye?
