Advertisements
Advertisements
Question
When a co-ordination compound CrCl3.6H2O is mixed with AgNO3, 2 moles of AgCl are precipitated per mole of the compound. Write
(i) Structural formula of the complex.
(ii) IUPAC name of the complex.
Solution
As two moles of AgCl are obtained; therefore, the structural formula would contain two Cl− ions satisfying the primary valencies, while five H2O molecules and one Cl−ion are present inside the coordination sphere, making the coordination number 6. One H2O molecule will be present as the molecule of hydration.
Structural formula: [CrCl(H2O)5]Cl2.H2O
IUPAC name of the compound: Pentaaquachloridochromium(III) chloride
APPEARS IN
RELATED QUESTIONS
Write the IUPAC name of [ Co(NO2)3(NH3)3 ].
Write the IUPAC name of the given compound:
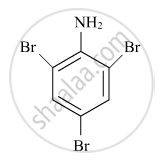
Write the IUPAC name of the given compound :
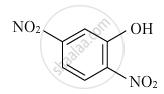
Write the IUPAC name of the following coordination compound:
[Co(NH3)6]Cl3
Write the IUPAC name of the following coordination compound:
[Co(NH3)5Cl]Cl2
Specify the oxidation number of the metal in the following coordination entity:
[Co(H2O)(CN)(en)2]2+
Using IUPAC norms write the systematic name of the following:
[Co(NH3)6]Cl3
How will you convert the following?
Aniline into N−phenylethanamide
Name the type of isomerism shown by the following compounds:
[CU(NH3)4] [PtCl4] and [Pt(NH3)4] [CuCl4]
Write the IUPAC name of the following complex:
[CoBr2(en)2]+
