Advertisements
Advertisements
Question
Specify the oxidation number of the metal in the following coordination entity:
[Co(H2O)(CN)(en)2]2+
Solution
Let the oxidation number of the metal be x.
Hence, x + 0 + (−1) + (2 × 0) = +2
x = 2 + 1
∴ x = +3
APPEARS IN
RELATED QUESTIONS
What is the IUPAC name of 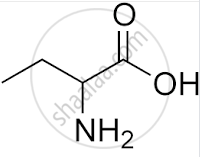
IUPAC name of K4[Fe(CN)6] is
Write the IUPAC name of the given compound :
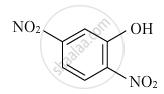
Write down the IUPAC name of the following complex: [Cr(NH3)2Cl2(en)]Cl (en = ethylenediamine)
Write the IUPAC name of the following coordination compound:
K2[PdCl4]
Write the IUPAC name of the following coordination compound:
[Pt(NH3)2Cl(NH2CH3)]Cl
Specify the oxidation number of the metal in the following coordination entity:
[CoBr2(en)2]+
Using IUPAC norms write the systematic name of the following:
[Pt(NH3)2Cl(NH2CH3)]Cl
Using IUPAC norms, write the systematic name of the following:
[Co(NH3)4Cl(NO2)]Cl
Using IUPAC norms, write the systematic name of the following:
[NiCl4]2−
Using IUPAC norms, write the systematic name of the following:
[Ni(NH3)6]Cl2
Using IUPAC norms, write the systematic name of the following:
[Co(en)3]3+
Write down the IUPAC name of the following complex and indicate the oxidation state, electronic configuration and coordination number. Also, give the stereochemistry and magnetic moment of the complex:
[Co(NH3)5Cl]Cl2
Write down the IUPAC name of the following complex and indicate the oxidation state, electronic configuration and coordination number. Also, give the stereochemistry and magnetic moment of the complex:
K4[Mn(CN)6]
Identify 'A' and 'B' and rewrite the reactions

Write the IUPAC names of the following coordination compounds:
[CoBr2(en)2]+, (en = ethylenediamine)
How will you convert the following?
Aniline into N−phenylethanamide
Name the type of isomerism shown by the following compounds:
[Co(Pn)2Cl2]+ and [Co(en)2Cl2]+
