Advertisements
Advertisements
Question
Which of the following alkenes on ozonolysis give a mixture of ketones only?
| (i) | CH3 – CH = CH – CH3 |
| (ii) | \[\begin{array}{cc} \ce{CH3 - C - CH = CH2}\\ |\phantom{.......}\\ \ce{CH3}\phantom{....} \end{array}\] |
| (iii) | 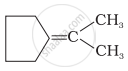 |
| (iv) | \[\begin{array}{cc} \phantom{...................}\ce{CH3}\\ \phantom{..............}/\\ \ce{(CH3)2 C = C}\\ \phantom{..............}\backslash\\ \phantom{...................}\ce{CH3} \end{array}\] |
Solution
| (iii) |  |
| (iv) | \[\begin{array}{cc} \phantom{...................}\ce{CH3}\\ \phantom{..............}/\\ \ce{(CH3)2 C = C}\\ \phantom{..............}\backslash\\ \phantom{...................}\ce{CH3} \end{array}\] |
Explanation:
Alkenes which have two substituents on each carbon atom of the double bond give a mixture of ketones on ozonolysis. Thus, options (iii) and (iv) give mixture of ketones.
(iii)
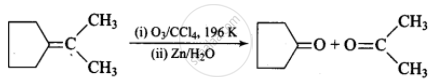
(iv)
\[\begin{array}{cc}
\phantom{....................}\ce{CH3}\phantom{..................................}\ce{CH3}\phantom{}\\
\phantom{...............}/\phantom{.....................................}/\phantom{}\\
\ce{(CH3)2 C = C ->[(i) O3/CCl4, 196 K][(ii) Zn/H2O] (CH3)2 C = O + O = C}\\
\phantom{...............}\backslash\phantom{.....................................}\backslash\\
\phantom{....................}\ce{CH3}\phantom{..................................}\ce{CH3}\phantom{}
\end{array}\]
On the other hand, alkenes (i) and (ii) give a mixture of two aldehydes.
(i) \[\ce{CH3CH = CH - CH3 ->[O3/CCl4, 196 K][Zn/H2O] CH3CH = O + O = CHCH3}\]
(ii) \[\begin{array}{cc}
\ce{CH3 - CH - CH = CH2 ->[O3/CCl4, 196 K][Zn/H2O] CH3 - CH - CH = O + O = CH2}\\
\phantom{...}|\phantom{.....................................}|\phantom{....................}\\
\phantom{...}\ce{CH3}\phantom{.................................}\ce{CH3}\phantom{.................}
\end{array}\]
APPEARS IN
RELATED QUESTIONS
Write IUPAC name of the product obtained by the ozonolysis of the following compound.
Pent-2-ene
An alkene ‘A’ contains three C – C, eight C – H σ bonds and one C – C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
Write a chemical equation for combustion reaction of the following hydrocarbon:
Hexyne
Arrange the halogens F2, Cl2, Br2, I2, in order of their increasing reactivity with alkanes.
The addition of HBr to 1-butene gives a mixture of products A, B and C
| (A) | 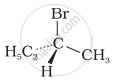 |
| (B) | 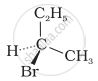 |
| (C) | CH3 – CH2 – CH2 – CH2 – Br |
The mixture consists of:
The major product formed in the following reactions is:
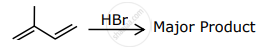
What would be the main product when propene reacts with HBr?
Propene, \[\ce{CH3 - CH = CH2}\] can be converted to 1-propanol by oxidation. Which set of reagents among the following is ideal to effect the conversion ______.
3-Methyl-pent-2-ene on reaction with HBr in presence of peroxide forms an addition product. The number of possible stereoisomers for the product is ______.
An alkene ‘A’ contains three C – C, eight C – H σ bonds and one C – C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write the IUPAC name of ‘A’.
