Advertisements
Advertisements
Question
Which of the following is not a straight chain hydrocarbon?
Options
\[\begin{array}{cc}
\ce{H3C - CH2 - CH2 - CH2 - CH2}\\
\phantom{.........................}|\\
\phantom{............................}\ce{CH3}
\end{array}\]\[\ce{H3C - CH2 - CH3 - CH2 - CH3}\]
\[\begin{array}{cc}
\ce{CH3}\phantom{................}\\
|\phantom{..................}\\
\ce{H2C - H2C - H2C - CH2}\\
\phantom{...................}|\\
\phantom{.....................}\ce{CH3}
\end{array}\]\[\begin{array}{cc}
\ce{CH3}\phantom{.............................}\\
\backslash\phantom{.........................}\\
\ce{CH - CH2 - CH2 - CH3}\\
/\phantom{.........................}\\
\ce{H3C}\phantom{.............................}\\
\end{array}\]
Solution
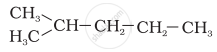
Explanation -
In the hydrocarbon (d), branching takes place at carbon-2.
APPEARS IN
RELATED QUESTIONS
Explain the following term with an example.
Functional group
Write the molecular formula of the given compound.
Acetylene
Write the molecular formula of the given compound.
Acetone
Write the molecular formula of the given compound.
Isobutane
In LPG, butane is a flammable component.
Explain the concept of heteroatoms with the help of examples.
Write a short note.
Catenation power
Write a short note.
Functional groups in carbon compounds
Match the following.
| Functional group –OH | - | Benzene |
| Heterocyclic | - | Potassium stearate |
| Unsaturated | - | Alcohol |
| Soap | - | Furan |
| Carbocyclic | - | Ethene |
Chlorine reacts with saturated hydrocarbons at room temperature in the
