Advertisements
Advertisements
Question
Why does the electrical conductivity of semiconductors increase with rise in temperature?
Solution
The gap between conduction band and valence band is small in semiconductors (Figure), therefore, some electrons from the valence band can jump to the conduction band and show some conductivity band. With rise in temperature more electrons can jump to the conduction band. Thus, electrical conductivity of semiconductors increases with rise in temperature.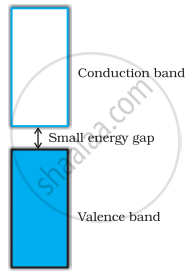
APPEARS IN
RELATED QUESTIONS
Non-stoichiometric cuprous oxide, Cu2O can be prepared in laboratory. In this oxide, copper to oxygen ratio is slightly less than 2:1. Can you account for the fact that this substance is a p-type semiconductor?
Classify the following as being either a p-type or an n-type semiconductor:
Ge doped with In
Explain the following with suitable examples:
12-16 and 13-15 group compounds.
What type of semiconductor is obtained when
Ge is doped with In?
Semiconductors have conductivity (in ohm-1 m-1) in the range of ____________.
Electrical conductivity of semiconductors increases with increase in ____________.
Which kind of defects are introduced by doping?
Silicon doped with electron-rich impurity forms ______.
Why are solids incompressible?
Silicon is a/an ______
