Advertisements
Advertisements
Question
With a labelled diagram, explain the electro-refining of a particular metal.
Solution
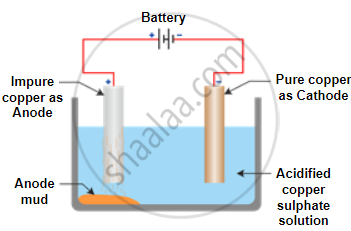
The process of electrolytic refining copper:
- In this electrolytic refining, the electrolyte is a solution of copper sulphate.
- In this process, the anode is impure copper, whereas the cathode is a thin strip of pure copper.
- On passing the current through the electrolyte, the pure copper from the anode dissolves into the electrolyte.
- An equivalent amount of pure copper from the electrolyte is deposited at the cathode.
- The insoluble impurities that settle down at the bottom of the anode (the positively charged electrode) are known as anode mud.
APPEARS IN
RELATED QUESTIONS
Metal A has an electronic configuration of 2, 8, 1 and metal B has 2, 8, 8, 2 which is a more reactive metal.
Give the effect of heat on their: nitrates
Name the processes involved in refining of ores.
Explain the following terms:
(c) slag
`ZnCO_3`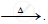 ..............................
..............................
`2AgNO_3` ...........................
...........................
Where are the cathode and anode in the electrolytic cell? Name the material used for these?
state the reactions at the two electrodes ?
Which metal is used for:
making face creams
Which of following metals occurs in native state?
A process of extracting metals from aqueous solutions of their salts using suitable reducing agents is called ______
