Advertisements
Advertisements
Question
Write a balanced equation for the following word equation:
Potassium bromide + Chlorine → Potassium chloride + Bromine
Solution
2KBr + Cl2 → 2KCl + Br2
APPEARS IN
RELATED QUESTIONS
State one characteristic of the chemical reaction which takes place when lemon juice is added gradually to potassium permanganate solution.
Balance the following chemical equation:
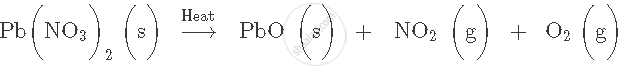
Balance the following equation. Also name the product formed.
CO2 + C → CO
Balance the following equation:
NaHCO3 → Na2CO3 + H2O + CO2
Write the balanced chemical equation of the following reaction. potassium bicarbonate + sulphuric acid → potassium sulphate + carbon dioxide + water
A chemical reaction is generally accompanied by certain external indications or characteristics. These include – change of – (a) colour (b) state (c) smell (d) evolution of gas (e) formation of precipitate (f) evolution or absorption of heat. With reference to change of colour – state the change in colour seen when the following are heated – copper carbonate.
A chemical reaction is generally accompanied by certain external indications or characteristics. These include – change of – (a) colour (b) state (c) smell (d) evolution of gas (e) formation of precipitate (f) evolution or absorption of heat. With reference to change of colour – state the change in colour seen when the following are heated – zinc carbonate.
In certain reaction an insoluble solid called precipitate is formed. State the colour and name of the precipitate formed in the following reaction involving addition of:
Iron [II] sulphate to sodium hydroxide.
Balance the following simple equation:
K + CO2 → K2O + C
Complete the following blank in the equation as indicated.
\[\ce{CaH2_{(s)} + 2H2O_{( aq)}-> Ca(OH)2_{(s)} + 2H2_{(g)}}\]
Grams: 42 g + ______ `→` ______ + ______
