Advertisements
Advertisements
Question
Write a short note.
Specific heat capacity
Solution
- The amount of heat energy required to raise the temperature of a unit mass of an object by 1 °C is called the specific heat or specific heat capacity of that object.
- SI unit of specific heat capacity is J/kg °C and CGS unit is cal/g °C
- It is denoted by ‘c’ and given by formula: c = `"Q"/("m"Delta "T")`
- Specific heat is a property of a substance and is different for different substances.
- Higher the value of specific heat capacity of the substance, higher is the amount of heat energy required to raise its temperature by 1 °C.
- The specific heat capacity of object is measured using principle of heat exchange.
RELATED QUESTIONS
The temperature of 170 g of water at 50°C is lowered to 5°C by adding a certain amount of ice to it. Find the mass of ice added.
Given: Specific heat capacity of water = 4200 J kg-1 °C-1 and specific latent heat of ice = 336000 J kg-1.
Why do the farmers fill their fields with water on a cold winter night?
In an experiment to determine the specific heat capacity of a solid following operations were
made:
Mass of calorimeter + stirrer = x kg
Mass of water = y kg
Initial temperature of water t1℃
Mass of solid = z kg
Temperature of solid = t2 ℃
Temperature of mixture = t ℃
Specific heat capacity of calorimeter and water are c1 and c2 respectively. Express the specific
heat capacity c of the solid in terms of the above data.
Explain the term boiling ?
Name the radiations for which the green house gases are opaque ?
Give three reasons for the increase of green house gases.
State the impact of global warming on life on the earth.
What is the specific heat capacity of boiling water?
What change in heat energy occurs when lead at its melting point
solidifies without change in the temperature?
An equal quantity of heat is supplied to two substances A and B. The substance A shows a greater rise in temperature. What can you say about the heat capacity of A as compared to that of B?
Discuss how high specific heat capacity of water helps in formation of land and sea breeze.
Will the value of specific heat’capacity and specific latent heat of a substance change if the scale is °F instead of °C?
Explain how heat capacity of a solid can be determined by the method of mixture.
A diatomic gas undergoes adiabatic change. Its pressure 'P' and temperature 'T' are related as p ∝ Tx, where x is ______.
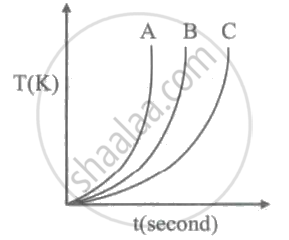
Which of the following substances (A, B and C) has the highest specific beat?
An office room contains about 4000 moles of air. The change in the internal energy of this much air when it is cooled from 34° C to 19° C at a constant pressure of 1.0 atm is (Use `gamma_"air"` = 1.4 and Universal gas constant = 8.314 J / mol K) ____________.
Two metals A and B have specific heat capacities in the ratio 2 : 3. If they are supplied the same amount of heat then
Which metal piece will show a greater rise in temperature given their masses is the same?
J/Kg °C is the unit of specific heat capacity.
