Advertisements
Advertisements
Question
Write one balanced equation to show Emission of `beta^-` (i.e. a negative beta particle)
Solution
Emission of `beta^-` (i.e. a negative beta particle)
When an energetic `gamma` - ray photon falls on heavy substance, it is absorbed by some nucleus of the substance, and its energy gives rise to the production of an electron and a position. This phenomenon in which energy is converted into mass is called pair production.
Equation :
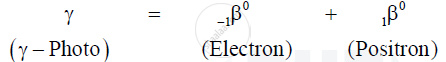
APPEARS IN
RELATED QUESTIONS
Two stable isotopes of lithium `""_3^6"Li"` and `""_3^7"Li"` have respective abundances of 7.5% and 92.5%. These isotopes have masses 6.01512 u and 7.01600 u, respectively. Find the atomic mass of lithium.
The nucleus `""_10^23"Ne"` decays by `beta^(-)`emission. Write down the β decay equation and determine the maximum kinetic energy of the electrons emitted. Given that:
`"m"(""_10^23 "Ne")` = 22.994466 u
`"m"(""_11^23 "Na")` = 22.989770 u.
With the help of a suitable example and an equation, explain the term pair production.
The mass number of a nucleus is
The atomic mass of Uranium `""_92^238"U"` is 238.0508 u, while that of Thorium `""_90^234"Th"` is 234.0436u, and that of Helium `""_2^4"He"` "is 4.0026u. Alpha decay converts `""_92^238"U"` into `""_92^234"Th"` as, shown below:
`""_92^238"U" -> ( ""_90^234"Th" + ""_2^4"He" + "Energy" )`
Find the Q-value and the kinetic energy of the emitted α-particle in the α-decay of `""_86^220"Rn"`.
Given `"m"(""_88^226"Ra")` = 226.02540 u, `"m"(""_86^222 "Rn")` = 222.01750 u,
`"m"(""_86^220 "Rn")`= 220.01137 u, `"m"(""_84^216 "Po")`= 216.00189 u.
\[\ce{^197_79Au}\] contains ______.
Distinguish between isotopes and isobars.
Two nuclei have different mass numbers A1 and A2. Are these nuclei necessarily the isotopes of the same element? Explain.
What conclusion is drawn from Rutherford’s scattering experiment of α-particles?
