Advertisements
Advertisements
Question
Write the chemical formula of the following compound in a step-by-step manner –
Aluminium hydroxide
Solution
Write the formula of
| Aluminium hydroxide | Symbol | Valency |
| Aluminium | \[\ce{Al}\] | \[\ce{3^{+}}\] |
| Hydroxide | \[\ce{OH}\] | \[\ce{1^{-}}\] |
Step I – Write each symbol with its valency.
Positive ion is written first
\[\ce{Al}^{3+}\] \[\ce{[OH]}^{1-}\]
Step II – Interchange the valencies positive ion is written first.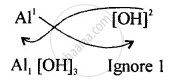
The Formula is \[\ce{Al[OH]3}\]
APPEARS IN
RELATED QUESTIONS
Define of Molecular formula.
Write the molecular formula for the oxide and sulphide of following elements of Calcium
Write the molecular formula for the oxide and sulphide of following elements of Hydrogen
Write the molecular formulae for the following compounds and name the elements present of Sulphuric acid
The valency of aluminium is 3. Write the valency of other radicals present in the following compounds of Aluminium chloride.
Write the molecular formulae of Magnesium carbonate.
Copper having electronic configuration 2,8,18,1 exhibits variable valency. Give a reason for the same and name the compound CuCI and CuCl2.
Write the chemical formula of the following compound in a step-by-step manner –
Magnesium nitride
Write the chemical formula of the following compound in a step-by-step manner –
Copper [II] oxide
Match the statements – 1 to 10 below with their correct answers from – A to J.
| Column I | Column II |
| 1. Elements having valency of two. | A: Br1− |
| 2. An anion | B: Divalent |
| 3. A gaseous non-metal | C: Reactants |
| 4. A cation. | D: Ammonium |
| 5. The term used for the substances which take part in the chemical reaction | E: Nitric oxide |
| 6. The meaning of the symbol ‘Δ’ over the arrow in a chemical equation. | F: Nitrogen |
| 7. The chemical name for nitrogen monoxide. | G: Zero |
| 8. A radical containing nitrogen and hydrogen only | H: Nitrous oxide |
| 9. The chemical name for dinitrogen oxide | I: Heat required |
| 10. The valency of noble gases | J: K1+ |
