Advertisements
Advertisements
Question
Write the chemical formula of the following compound in a step-by-step manner –
Copper [II] oxide
Solution
Write the formula of
| Copper [II] oxide | Symbol | Valency |
| Copper | \[\ce{Cu}\] | \[\ce{2^{+}}\] |
| Oxide | \[\ce{O}\] | \[\ce{2^{-}}\] |
Step I – Write each symbol with its valency.
Positive ion is written first
\[\ce{Cu}^{2+}\] \[\ce{O}^{2-}\]
Step II – Interchange the valencies ignoring (+) and (−) signs.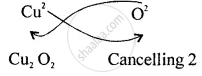
The Formula is CuO
APPEARS IN
RELATED QUESTIONS
Define of Molecular formula.
Write the molecular formula for the oxide and sulphide of following elements of Calcium
Write the molecular formulae for the following compounds and name the elements present of Baking soda.
A solution of a substance ‘X’ is used for white washing.
(a) Name the substance ‘X’ and write its formula.
(b) Write the reaction of the substance ‘X’ named in (a) above with water.
Write the molecular formulae of Magnesium carbonate.
Write the chemical formula of the following compound in a step-by-step manner –
Calcium bicarbonate
Write the chemical formula of the following compound in a step-by-step manner –
Sodium bisulphate
Write the chemical formula of the following compound in a step-by-step manner –
Sodium zincate
Write the chemical formula of the following compound in a step-by-step manner –
Iron [II] hydroxide
Complete the statement given below by filling in the blanks with the correct words.
The valency of iron in FeO is _______ of chlorine [chloride] in CaCl2 is ________ and of dichromate in K2 Cr2 O7 is ________.
