Advertisements
Advertisements
Question
Write the equation of the following reaction:
Bromination of anisole in an ethanoic acid medium.
Solution
Phenylalkyl ethers undergo the usual halogenation in the benzene ring; e.g., anisole undergoes bromination with bromine in ethanoic acid even in the absence of iron (III) bromide catalyst. It is due to the activation of the benzene ring by the methoxy group. Para isomer is obtained in 90% yield.

APPEARS IN
RELATED QUESTIONS
Predict the product of the following reaction:
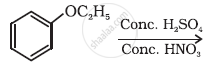
Write the equation of the following reaction:
Friedel-Crafts reaction - alkylation of anisole
Write the equation of the following reaction:
Nitration of anisole.
Write the equation of the following reaction:
Friedel-Craft’s acetylation of anisole.
What happens when Anisole is treated with CH3Cl/anhydrous AlCl3?
Why phenol undergoes electrophilic substitution more easily than benzene?
Anisole is called ____________.
An ether is more volatile than alcohol having the same molecular formula. This is due to:
Write the equation for the following:
Reaction of chlorobenzene with CH3Cl/anhyd. AlCl3.
Anisole undergoes bromination with bromine in ethanoic acid even in the absence of iron (III) bromide catalyst.
