Advertisements
Advertisements
Question
Write the names and formulas of the products formed when methane reacts with chlorine. Write the chemical equations.
Solution
Substitution reaction takes place.
Methane with Chlorine:
\[\ce{CH4 + Cl2 ->[Sunlight] \underset{Methyl chloride}{CH3Cl} + HCl}\]
or
1.
Methyl chloride
\[\begin{array}{cc}
\ce{H}\phantom{....................}\ce{H}\phantom{.......}\\
|\phantom{.....................}|\phantom{.......}\\
\ce{H - C - H + Cl2 -> H - C - Cl + HCl}\\
|\phantom{.....................}|\phantom{.......}\\
\ce{H}\phantom{....................}\ce{H}\phantom{.......}\\
\end{array}\]
2. Dichloro methane
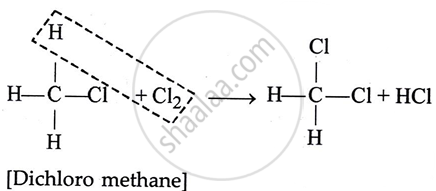
3. Chloroform or [Tri chlorornethane]
\[\begin{array}{cc}
\ce{Cl}\phantom{....................}\ce{Cl}\phantom{.......}\\
|\phantom{......................}|\phantom{.......}\\
\ce{Cl - C - H + Cl2 -> Cl - C - Cl + HCl}\\
|\phantom{......................}|\phantom{.......}\\
\ce{H}\phantom{.....................}\ce{H}\phantom{.......}\\
\end{array}\]
4. Carbon-tetrachloride
\[\begin{array}{cc}
\ce{Cl}\phantom{...................}\ce{Cl}\phantom{.......}\\
|\phantom{.....................}|\phantom{.......}\\
\ce{Cl - C - H + Cl2 -> Cl - C - Cl + HCl}\\
|\phantom{.....................}|\phantom{.......}\\
\ce{H}\phantom{....................}\ce{Cl}\phantom{.......}\\
\end{array}\]
RELATED QUESTIONS
Classify the compound C3H4 as alkanes, alkenes, and alkynes.
Classify the compound C3H8 as alkanes, alkenes, and alkynes.
Methane is a greenhouse gas. comment.
Write the molecular formula of methane.
How is ethane prepared in the laboratory?
Conversion of ethanol to ethene by the action of concentrated sulphuric acid is an example of _________.
Name the saturated hydrocarbon containing two carbon atoms.
Which type of reaction will Ethene undergo?
Write the IUPAC name of the following :
\[\begin{array}{cc}
\phantom{....}\ce{H}\phantom{...} \ce{H} \phantom{...}\ce{H}\phantom{....}\\
\phantom{..}|\phantom{....}|\phantom{....}|\phantom{..}\\
\ce{H - C - C = C - H}\\
\phantom{..........}|\\
\phantom{..........} \ce{H}
\end{array}\]
Write the IUPAC name of the following :
\[\begin{array}{cc}
\phantom{.}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\\
|\phantom{....}|\phantom{....}|\\
\ce{H - C = C - C - H}\\
\phantom{.........}|\\
\phantom{.........}\ce{H}\\
\end{array}\]
