Advertisements
Advertisements
Question
You can buy solid air-freshners in shops. Do you think these substance are ionic or covalent? Why?
Solution
These substances are covalent in nature because bonding between covalent substances are not as strong as ionic substances. So, covalent compounds hold together with weak forces and low melting point. This is why, they vapourise easily and give their odours. However, when ionic compounds comes in contact with air, they cannot give an odour by vapourising.
APPEARS IN
RELATED QUESTIONS
Elements forming ionic compounds attain noble gas configuration by either gaining or losing electrons from their outermost shells. Give reason to explain why carbon cannot attain noble gas configuration in this manner to form its compounds.
Write any three features and give two examples of covalent compounds
What type of bonds are present in methane (CH4) and sodium chloride (NaCl)?
Give one example of a molecule containing a single covalent bond.
Using electron-dot diagrams which show only the outermost shell electrons, show how a molecule of nitrogen, N2, is formed from two nitrogen atoms. What name is given to this type of bonding? (Atomic number of nitrogen is 7)
Draw the electron-dot structure of HCl compound and state the type of bonding.
What is the difference between ionic compounds and covalent compounds?
The following structural formula belongs to which carbon compound?
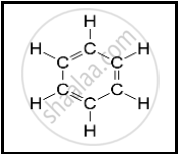
Choose and write the correct option.
Which type of carbon-carbon bonds are present in Vanaspati ghee?
Explain the following briefly:
Cl2 is a non polar molecule, while HCl is a polar molecule.
(a) Compound X consists of molecules.
Choose the letter corresponding to the correct answer from the choices (a), (b), (c) and (d) given below
X is likely to have a :
Compare the compounds carbon tetrachloride and sodium chloride with regard to solubility in water and electrical conductivity.
The molecular masses of a carbon compound spread over a range of _______.
Write a short note.
Aromatic hydrocarbons
Complete the following activity.
Write the names of the hydrocarbons for the following structural formula.
(isobutylene, cyclohexane, propene, cyclohexene, cyclopentane, benzene, propyne, isobutane, propane)
| \[\begin{array}{cc}\phantom{......}\ce{H}\phantom{...}\ce{H}\phantom{...}\ce{H}\phantom{..}\\ \phantom{.....}|\phantom{....}|\phantom{....}|\\ \ce{H - C - C = C}\\\phantom{.....}|\phantom{.........}|\\ \phantom{.....}\ce{H}\phantom{........}\ce{H}\end{array}\] |
Consider the coordination compound, K2[Cu(CN)4]. A coordinate covalent bond exists between:
______ is an example of a covalent compound having a high melting point.
In covalent bond formation, the sharing of ______ electrons takes place in their outermost shell.
