English Medium
Academic Year: 2017-2018
Date: March 2018
Advertisements
A Mendelian experiment consisted of breeding pea plants bearing violet flowers with pea plants bearing white flowers. What will be the result in F1 progeny?
Chapter: [0.08] Heredity
Write the energy conversion that takes place in a hydropower plant.
Chapter: [0.14] Sources of Energy
A compound 'X' on heating with excess conc. sulphuric acid at 443 K gives an unsaturated compound 'Y'. 'X' also reacts with sodium metal to evolve a colourless gas 'Z'. Identify 'X', 'Y' and 'Z'. Write the equation of the chemical reaction of formation of 'Y' and also write the role of sulphuric acid in the reaction.
Chapter: [0.04] Carbon and its Compounds
Name one gustatory receptor and one olfactory receptor present in human beings.
Chapter: [0.06] Control and Co-ordination
Write a and b in the given flow chart of the neuron through which information travels as an electrical impulse.
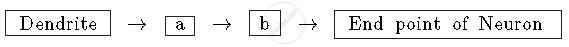
Chapter: [0.06] Control and Co-ordination
If the image formed by a spherical mirror for all positions of the object placed in front of it is always erect and diminished, what type of mirror is it? Draw a labelled ray diagram to support your answer.
Chapter: [0.09] Light - Reflection and Refraction
Decomposition reactions require energy either in the form of heat or light or electricity for breaking down the reactants. Write one equation each for decomposition reactions where energy is supplied in the form of heat, light and electricity
Chapter: [0.01] Chemical Reactions and Equations
2 mL of sodium hydroxide solution is added to a few pieces of granulated zinc metal taken in a test tube. When the contents are warmed, a gas evolves which is bubbled through a soap solution before testing. Write the equation of the chemical reaction involved and the test to detect the gas. Name the gas which will be evolved when the same metal reacts with dilute solution of a strong acid.
Chapter: [0.01] Chemical Reactions and Equations
The pH of a salt used to make tasty and crispy pakoras is 14. Identify the salt and write a chemical equation for its formation. List its two uses.
Chapter: [0.02] Acids, Bases and Salts
Why are most carbon compounds poor conductors of electricity?
Chapter: [0.03] Metals and Non Metals [0.04] Carbon and its Compounds
Write the name and structure of a saturated compound in which the carbon atoms are arranged in a ring. Give the number of single bonds present in this compound.
Chapter: [0.04] Carbon and its Compounds
Name the hormones secreted by the given endocrine glands and specify one function.
Thyroid
Chapter: [0.06] Control and Co-ordination
Name the hormones secreted by the given endocrine glands and specify one function.
Pituitary
Chapter: [0.06] Control and Co-ordination
Name the hormones secreted by the given endocrine glands and specify one function.
Pancreas
Chapter: [0.06] Control and Co-ordination
Write one difference between asexual and sexual mode of reproduction.
Chapter: [0.07] How do Organisms Reproduce?
Which species is likely to have better chances of survival - the one reproducing asexually or the one reproducing sexually? Justify your answer.
Chapter: [0.07] How do Organisms Reproduce?
Advertisements
Explain the term absolute refractive index of a medium and write an expression to relate it with the speed of light in vacuum.
Chapter: [0.09] Light - Reflection and Refraction
What is meant by a power of a lens? Define its SI unit.
Chapter: [0.09] Light - Reflection and Refraction
A student uses a lens of focal length 40 cm and another of –20 cm. Write the nature and power of each lens
Chapter: [0.09] Light - Reflection and Refraction
Show how would you join three resistors, each of resistance 9 Ω so that the equivalent resistance of the combination is
1) 13.5
2) 6 Ω
Chapter: [0.11] Electricity
Write Joule's law of heating.
Chapter: [0.11] Electricity
Two lamps, one rated 100 W at 220 V, and the other 60 W at 220 V, are connected in parallel to electric mains supply. What current is drawn from the line if the supply voltage is 220 V?
Chapter: [0.11] Electricity
List the factors on which the resistance of a conductor in the shape of a wire depends.
Chapter: [0.11] Electricity
Why are metals good conductors of electricity whereas glass is a bad conductor of electricity? Give reason?
Chapter: [0.11] Electricity
Why are alloys commonly used in electrical heating devices? Give reason.
Chapter: [0.11] Electricity
Students in a school listened to the news read in the morning assembly that the mountain of garbage in Delhi suddenly exploded and various vehicles got buried under it. Several people were also injured and there was traffic jam all around. In the brain storming session the teacher also discussed this issue and asked the students to find out a solution to the problem of garbage. Finally they arrived at two main points -one is self management of the garbage we produce and the second is to generate less garbage at individual level.
1) Suggest two measures to manage the garbage we produce.
2) As an individual, what can we do to generate the least garbage? Give two points.
3) List two values the teacher instilled in his student in this episode.
Chapter: [0.13] Our Environment [0.16] Sustainable Management of Natural Resources
What is a dam? Why do we seek to build large dams? While building large dams, which three main problems should particularly be addressed to maintain peace among local people? Mention them.
Chapter: [0.16] Sustainable Management of Natural Resources
Write the steps involved in the extraction of pure metals in the middle of the activity series from their carbonate ores.
Chapter: [0.03] Metals and Non Metals
How is copper extracted from its sulphide ore? Explain the various steps supported by chemical equations. Draw labelled diagram for the electrolytic refining of copper.
Chapter: [0.03] Metals and Non Metals
The modern periodic table has been evolved through the early attempts of Dobereiner, Newland and Mendeleev. List one advantage and one limitation or all the three attempts.
Chapter: [0.05] Periodic Classification of Elements
Name the scientist who first of all showed that atomic number of an element is a more fundamental property than its atomic mass.
Chapter: [0.05] Periodic Classification of Elements
State modern periodic law.
Chapter: [0.05] Periodic Classification of Elements
Mention any two components of blood.
Chapter: [0.05] Life Processes [0.05] Life Processes
Trace the movement of oxygenated blood in the body.
Chapter: [0.05] Life Processes [0.05] Life Processes
Write the function of valves present in between atria and ventricles.
Chapter: [0.05] Life Processes
Write one structural difference between the composition of arteries and veins.
Chapter: [0.05] Life Processes
Define excretion
Chapter: [0.05] Life Processes [0.05] Life Processes
Name the basic filtration unit present in the kidney.
Chapter: [0.05] Life Processes [0.05] Life Processes
Draw the well-labelled diagram of the human excretory system
Chapter: [0.05] Life Processes [0.05] Life Processes
Advertisements
Write the functions of the following parts in human female reproductive system:-
(i) Ovary
(ii) Oviduct
(iii) Uterus
Chapter: [0.07] How do Organisms Reproduce?
Describe in brief the structure of placenta
Chapter: [0.07] How do Organisms Reproduce?
Describe the function of placenta.
Chapter: [0.07] How do Organisms Reproduce?
A student is unable to see clearly the words written on the black board placed at a distance of approximately 3 m from him. Name the defect of vision the boy is suffering from. State the possible causes of this defect and explain the method of correcting it.
Chapter: [0.1] The Human Eye and the Colourful World
What is atmospheric refraction? Use this phenomenon to explain the following natural events:
Twinkling of stars
Draw diagrams to illustrate your answers.
Chapter: [0.1] The Human Eye and the Colourful World
Write the function of the following part of the human eye: Cornea
Chapter: [0.1] The Human Eye and the Colourful World
Write the function of the following part of the human eye:- iris
Chapter: [0.1] The Human Eye and the Colourful World
Write the function of the following part of the human eye: crystalline lens
Chapter: [0.1] The Human Eye and the Colourful World
Write the function of the following part of the human eye: ciliary muscles
Chapter: [0.1] The Human Eye and the Colourful World
Why does the sun appear reddish early in the morning? Will this phenomenon be observed by an observer on the moon? Justify your answer with a reason.
Chapter: [0.1] The Human Eye and the Colourful World
State Fleming's left hand rule.
Chapter: [0.12] Magnetic Effects of Electric Current
What is the Principle of an Electric motor?
Chapter: [0.12] Magnetic Effects of Electric Current
Explain the function of the following parts of an electric motor.
Armature
Chapter: [0.12] Magnetic Effects of Electric Current
Explain the function of the following parts of an electric motor.
Brushes
Chapter: [0.12] Magnetic Effects of Electric Current
Explain the function of the following parts of an electric motor.
Split ring
Chapter: [0.12] Magnetic Effects of Electric Current
A student added few pieces of aluminium metal to two test tubes A and B containing aqueous solutions of iron sulphate and copper sulphate. In the second part of her experiment, she added iron metal to another test tubes C and D containing aqueous solutions of aluminium sulphate and copper sulphate.
In which test tube or test tubes will she observe colour change? On the basis of this experiment, state which one is the most reactive metal and why.
Chapter: [0.03] Metals and Non Metals
What is observed when a solution of sodium sulphate is added to a solution of barium chloride taken in a test tube? Write equation for the chemical reaction involved and name the type of reaction in this case.
Chapter: [0.01] Chemical Reactions and Equations
List the steps of preparation of temporary mount of a leaf peel to observe stomata.
Chapter: [0.05] Life Processes
Name the process by which an Amoeba reproduces. Draw the various stages of its reproduction in a proper sequence.
Chapter: [0.07] How do Organisms Reproduce?
A student in viewing under a microscope a permanent slide showing various stages of asexual reproduction by budding in yeast. Draw diagrams of what he observes (in proper sequence)
Chapter: [0.07] How do Organisms Reproduce?
An object of height 4.0 cm is placed at a distance of 30 cm from the optical centre 'O' of a convex lens of focal length 20 cm. Draw a ray diagram to find the position and size of the image formed. Mark optical centre 'O' and principal focus 'F' on the diagram. Also find the approximate ratio of size of the image to the size of the object.
Chapter: [0.09] Light - Reflection and Refraction
The values of current (I) flowing through a given resistor of resistance (R), for the corresponding values of potential difference (V) across the resistor are as given below:
| V (volts) | 0.5 | 1.0 | 1.5 | 2.0 | 2.5 | 3.0 | 4.0 | 5.0 |
| I (amperes) | 0.1 | 0.2 | 0.3 | 0.4 | 0.5 | 0.6 | 0.8 | 1.0 |
Plot a graph between current (I) and potential difference (V) and determine the resistance (R) of the resistor.
Chapter: [0.11] Electricity
Other Solutions
Submit Question Paper
Help us maintain new question papers on Shaalaa.com, so we can continue to help studentsonly jpg, png and pdf files
CBSE previous year question papers Class 10 Science with solutions 2017 - 2018
Previous year Question paper for CBSE Class 10 Science-2018 is solved by experts. Solved question papers gives you the chance to check yourself after your mock test.
By referring the question paper Solutions for Science, you can scale your preparation level and work on your weak areas. It will also help the candidates in developing the time-management skills. Practice makes perfect, and there is no better way to practice than to attempt previous year question paper solutions of CBSE Class 10.
How CBSE Class 10 Question Paper solutions Help Students ?
• Question paper solutions for Science will helps students to prepare for exam.
• Question paper with answer will boost students confidence in exam time and also give you an idea About the important questions and topics to be prepared for the board exam.
• For finding solution of question papers no need to refer so multiple sources like textbook or guides.
