Advertisements
Advertisements
Construct a labelled diagram for the following cell:
`Zn|Zn^(2+)(1M)||H^+(1M)|H_(2(g,1atm))|Pt`
Concept: undefined > undefined
In the following
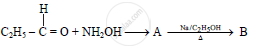
The compound ‘B’ is _______.
(A) Propan–1–amine
(B) Propan–2–amine
(C) Isopropylamine
(D) Dimethylamine
Concept: undefined > undefined
Advertisements
Identify A and B in the following reaction:
`CH_3-Br+Mg"dry ether"/""A+CO_2"dry ether"/(H^+/(H_2O))B+Mg(Br)OH`
Concept: undefined > undefined
Identify A and B in the following reaction:
`CH_3-Br+Mg"dry ether"/""A+CO_2"dry ether"/(H^+/(H_2O))B+Mg(Br)OH`
Concept: undefined > undefined
How ligands are classified? Explain with suitable examples.
Concept: undefined > undefined
Explain the following term
Flux
Concept: undefined > undefined
The group 15 element having inner electronic configuration as of argon is-
(a) Phosphorous (z = 15)
(b) Antimony (z =51)
(c) Arsenic (z = 33)
(d) Nitrogen (z = 7)
Concept: undefined > undefined
Explain any two chemical methods of food preservation.
Concept: undefined > undefined
In Van Arkel method of refining metal, impure zirconium is converted to unstable volatile
compound by heating it with _______.
(A) oxygen
(B) chlorine
(C) bromine
(D) iodine
Concept: undefined > undefined
Define isotonic solutions
Concept: undefined > undefined
Write electronic configuration and two uses of neon. (Z = 10)
Concept: undefined > undefined
Explain the mechanism of cleansing action of soaps.
Concept: undefined > undefined
Write balanced chemical equations for the action of hydrogen bromide on styrene in the presence of a peroxide
Concept: undefined > undefined
Write balanced chemical equations for the action of methyl bromide on silver propanoate
Concept: undefined > undefined
Write the electronic configuration of the following element:
Krypton (Z = 36)
Concept: undefined > undefined
Write cathode and anode reaction in a fuel cell.
Concept: undefined > undefined
The oxidation state of nitrogen in dinitrogen trioxide is ____
(a) + 1
(b) + 2
(c) + 3
(d) + 4
Concept: undefined > undefined
Baeyer’s reagent is -'
(a) acidified potassium dichromate
(b) alkaline potassium dichromate
(c) alkaline potassium permanganate
(d) acidified potassium permanganate
Concept: undefined > undefined
Why ethanol has the higher boiling point than ethane?
Concept: undefined > undefined
