Advertisements
Advertisements
प्रश्न
A compound 'A' of molecular formula C2H3OCl undergoes a series of reactions as shown below. Write the structures of A, B, C and D in the following reactions :
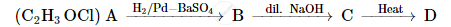
उत्तर
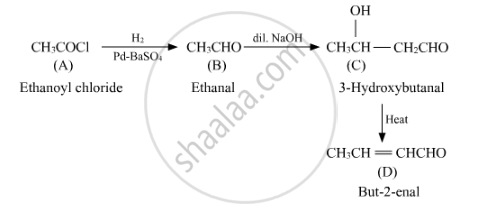
Using the given molecular formula, compound A is Ethanoyl Chloride CH3COCl, which undergoes reaction with poisoned palladium.
On carrying hydrogenation of A in the presence of poisoned palladium, we get an aldehyde. Hence, B can be Ethanal, CH3CHO.
An aldehyde, on treating with dilute alkali, undergoes aldol condensation reaction. Hence, C can be CH3CH(OH)CH2CHO.
On heating an aldol product, it loses water to produce a double bond and we get CH3CH=CHCHO.
Hence, we have
संबंधित प्रश्न
Write the products formed when CH3CHO reacts with the following reagents: CH3CHO in the presence of dilute NaOH
What is meant by the following term? Give an example of the reaction in the following case.
Aldol
How will you convert ethanal into the following compound?
But-2-enal
What is substituted imine called?
Which product is formed when the compound 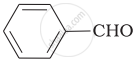 is treated with concentrated aqueous \[\ce{KOH}\] solution?
is treated with concentrated aqueous \[\ce{KOH}\] solution?
Why is there a large difference in the boiling points of butanal and butan-1-ol?
When liquid ‘A’ is treated with a freshly prepared ammoniacal silver nitrate solution, it gives bright silver mirror. The liquid forms a white crystalline solid on treatment with sodium hydrogensulphite. Liquid ‘B’ also forms a white crystalline solid with sodium hydrogensulphite but it does not give test with ammoniacal silver nitrate. Which of the two liquids is aldehyde? Write the chemical equations of these reactions also.
Which of the following gives aldol con~ensation reaction?
When acetaldehyde is treated with dilute NaOH, the following reaction is observed.
\[\begin{array}{cc}
\ce{2CH3 - CHO ->[dil.NaOH] CH3 - CH - CH2 - CHO}\\
\phantom{...............}|\\
\phantom{.................}\ce{OH}
\end{array}\]
- What are the functional groups in the product?
- Can another product be formed during the same reaction? (Deduce the answer by doing atomic audit of reactant and product).
- Is this an addition reaction or condensation reaction?
What is aldol condensation? Explain it with suitable examples.
