Advertisements
Advertisements
प्रश्न
A compound 'A' of molecular formula C2H3OCl undergoes a series of reactions as shown below. Write the structures of A, B, C and D in the following reactions :
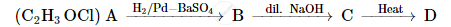
उत्तर
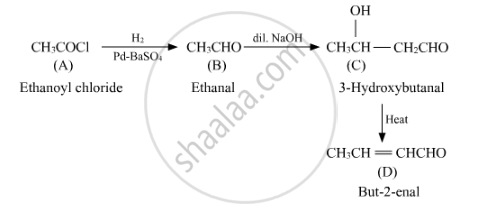
Using the given molecular formula, compound A is Ethanoyl Chloride CH3COCl, which undergoes reaction with poisoned palladium.
On carrying hydrogenation of A in the presence of poisoned palladium, we get an aldehyde. Hence, B can be Ethanal, CH3CHO.
An aldehyde, on treating with dilute alkali, undergoes aldol condensation reaction. Hence, C can be CH3CH(OH)CH2CHO.
On heating an aldol product, it loses water to produce a double bond and we get CH3CH=CHCHO.
Hence, we have
संबंधित प्रश्न
Write the products formed when CH3CHO reacts with the following reagents: CH3CHO in the presence of dilute NaOH
Which of the following compounds would undergo aldol condensation, which the Cannizzaro reaction and which neither? Write the structures of the expected products of aldol condensation and Cannizzaro reaction.
- Methanal
- 2-Methylpentanal
- Benzaldehyde
- Benzophenone
- Cyclohexanone
- 1-Phenylpropanone
- Phenylacetaldehyde
- Butan-1-ol
- 2, 2-Dimethylbutanal
Describe the following:
Cross aldol condensation
Write chemical equations of the following reaction :
Propanone is treated with dilute Ba (OH)2-.
Write chemical equations of the following reaction :
Benzoyl chloride is hydrogenated in the presence of `"Pd"/(BaSO_4)`
What is substituted imine called?
Cannizaro’s reaction is not given by ______.
Which product is formed when the compound 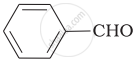 is treated with concentrated aqueous \[\ce{KOH}\] solution?
is treated with concentrated aqueous \[\ce{KOH}\] solution?
Which of the following compounds do not undergo aldol condensation?
(i) \[\ce{CH3 - CHO}\]
(ii) 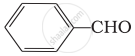
(iii) \[\begin{array}{cc}
\phantom{}\ce{O}\\
\phantom{}||\\
\ce{CH3 - C - CH3}
\end{array}\]
(iv) \[\begin{array}{cc}
\phantom{}\ce{CH3}\\
|\phantom{...}\\
\ce{CH3 - C - CHO}\phantom{..}\\
|\phantom{...}\\
\phantom{}\ce{CH3}\\
\end{array}\]
Give reasons to support the answer:
Presence of Alpha hydrogen in aldehydes and ketones is essential for aldol condensation.
