Advertisements
Advertisements
प्रश्न
A student was asked to perform an experiment in the laboratory based on the instructions given:
Observe the picture given below and state one observation for the experiment you would notice on mixing the given solution.
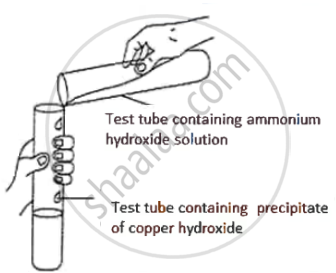
उत्तर
Blue ppt. dissolves to form an inky blue/deep blue solution.
APPEARS IN
संबंधित प्रश्न
Identify the substance underlined, in the following case
Cation that does not form a precipitate with ammonium hydroxide but forms one with sodium hydroxide
What do you observe when caustic soda solution is added to the following solution, first a little and then in excess:
ZnSO4
Write a balanced equation for this reaction.
What do you observe when caustic soda solution is added to the following solution, first a little and then in excess:
Pb(NO3)2
Write balanced equation for this reaction.
Name a white, insoluble oxide that dissolves when fused with caustic soda or caustic potash.
Identify the substance Q based on the information given below:
The white crystalline solid Q is soluble in water. It liberates a pungent smelling gas when heated with sodium hydroxide solution.
Choose the correct answer from the options given below :
Hydroxide of this metal is soluble is sodium hydroxide solution
Using Sodium hydroxide solution, how would you distinguish: Ammonium sulphate from sodium sulphate.
Sodium hydroxide solution is added to the solutions containing the ions mentioned in List X. List Y gives the details of the precipitate. Match the ions with their coloured precipitates.
| List X | List Y |
| (i) Pb2+ | (A) Reddish Brown |
| (ii) Fe2+ | (B) White insoluble inexcess |
| (iii) Zn2+ | (C) Dirty green |
| (iv) Fe3+ | (D) White soluble in excess |
| (v) Cu2+ | (E) White soluble in excess |
| (vi) Ca2+ | (F) Blue |
State your observation of the following case :
When excess sodium hydroxide is added to calcium nitrate solution
What is observed when hot, concentrated caustic soda solution is added to zinc? Write a balanced equation.
